Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine
- PMID: 18463198
- PMCID: PMC2562787
- DOI: 10.1124/dmd.107.019562
Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine
Abstract
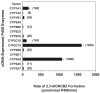
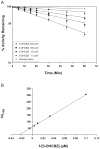
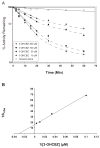
Conversion of the carbamazepine metabolite 3-hydroxycarbamazepine (3-OHCBZ) to the catechol 2,3-dihydroxycarbamazepine (2,3-diOHCBZ) followed by subsequent oxidation to a reactive o-quinone species has been proposed as a possible bioactivation pathway in the pathogenesis of carbamazepine-induced hypersensitivity. Initial in vitro phenotyping studies implicated CYP3A4 as a primary catalyst of 2,3-diOHCBZ formation: 2-hydroxylation of 3-OHCBZ correlated significantly (r(2) > or = 0.929, P < 0.001) with CYP3A4/5 activities in a panel of human liver microsomes (n = 14) and was markedly impaired by CYP3A inhibitors (>80%) but not by inhibitors of other cytochrome P450 enzymes (< or = 20%). However, in the presence of troleandomycin, the rate of 2,3-diOHCBZ formation correlated significantly with CYP2C19 activity (r(2) = 0.893, P < 0.001) in the panel of human liver microsomes. Studies with a panel of cDNA-expressed enzymes revealed that CYP2C19 and CYP3A4 were high (S50 = 30 microM) and low (S50 = 203 microM) affinity enzymes, respectively, for 2,3-diOHCBZ formation and suggested that CYP3A4, but not CYP2C19, might be inactivated by a metabolite formed from 3-OHCBZ. Subsequent experiments demonstrated that preincubation of 3-OHCBZ with human liver microsomes or recombinant CYP3A4 led to decreased CYP3A4 activity, which was both preincubation time- and concentration-dependent, but not inhibited by inclusion of glutathione or N-acetylcysteine. CYP3A4, CYP3A5, CYP3A7, CYP2C19, and CYP1A2 converted [14C]3-OHCBZ into protein-reactive metabolites, but CYP3A4 was the most catalytically active enzyme. The results of this study suggest that CYP3A4-dependent secondary oxidation of 3-OHCBZ represents a potential carbamazepine bioactivation pathway via formation of reactive metabolites capable of inactivating CYP3A4, potentially generating a neoantigen that may play a role in the etiology of carbamazepine-induced idiosyncratic toxicity.
Figures









References
-
- Bornheim LM, Correia MA. Selective inactivation of mouse liver cytochrome P-450IIIA by cannabidiol. Mol Pharmacol. 1990;38:319–326. - PubMed
-
- Bourdi M, Tinel M, Beaune PH, Pessayre D. Interactions of dihydralazine with cytochromes P4501A: a possible explanation for the appearance of anti-cytochrome P4501A2 autoantibodies. Mol Pharmacol. 1994;45:1287–1295. - PubMed
-
- Bu HZ, Kang P, Deese AJ, Zhao P, Pool WF. Human In Vitro Glutathionyl And Protein Adducts Of Carbamazepine-10,11-Epoxide A Stable And Pharmacologically Active Metabolite Of Carbamazepine. Drug Metab Dispos. 2005;30:1920–1924. - PubMed
-
- Cuttle L, Munns AJ, Hogg NA, Scott JR, Hooper WD, Dickinson RG, Gillam EM. Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab Dispos. 2000;28:945–950. - PubMed
-
- Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

