Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2
- PMID: 18463240
- PMCID: PMC6670728
- DOI: 10.1523/JNEUROSCI.0689-08.2008
Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2
Abstract
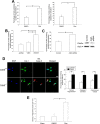
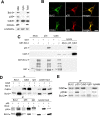
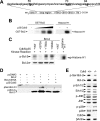
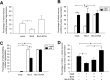
Accumulating evidence indicates that deregulation of cyclin-dependent kinase 5 (Cdk5) activity is associated with apoptosis in various neurodegenerative disease models. Interestingly, recent studies suggest that Cdk5 may also favor neuronal survival. Nonetheless, whether Cdk5 is directly required for neuronal survival during development remains enigmatic. In the current study, we established the pivotal role of Cdk5 in neuronal survival during development by demonstrating that reduction or absence of Cdk5 activity markedly exacerbated neuronal death in cultures and in vivo. Interestingly, the antiapoptotic protein Bcl-2 (B-cell lymphoma protein 2) was identified as a novel substrate of Cdk5. We found that Cdk5-mediated phosphorylation of Bcl-2 at Ser70 was required for the neuroprotective effect of Bcl-2. Intriguingly, inhibition of this phosphorylation conferred proapoptotic property to Bcl-2. Furthermore, overexpression of a Bcl-2 mutant lacking the Cdk5 phosphorylation site abolished the protective effect of Cdk5 re-expression in Cdk5(-/-) neurons, suggesting that Ser70 phosphorylation of Bcl-2 contributed to Cdk5-mediated neuronal survival. Our observations revealed that Cdk5-mediated Bcl-2 phosphorylation is pivotal for the antiapoptotic effect of Bcl-2 and contributes to the maintenance of neuronal survival by Cdk5. Our study has also identified Cdk5 as a regulator of Bcl-2 function in neuronal apoptosis.
Figures
References
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Blagosklonny MV. Unwinding the loop of Bcl-2 phosphorylation. Leukemia. 2001;15:869–874. - PubMed
-
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. - PubMed
-
- Cheung ZH, Ip NY. Cdk5: mediator of neuronal death and survival. Neurosci Lett. 2004;361:47–51. - PubMed
-
- Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, So KF. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci. 2004;25:383–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
