Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model
- PMID: 18463260
- PMCID: PMC6670746
- DOI: 10.1523/JNEUROSCI.4476-07.2008
Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model
Abstract
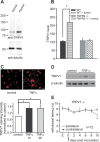
To provide a tool to investigate the mechanisms inducing and maintaining cancer-related pain and hyperalgesia, a soft tissue tumor/metastasis model was developed that is applicable in C57BL/6J wild-type and transgenic mice. We show that the experimental tumor-induced heat hyperalgesia and nociceptor sensitization were prevented by systemic treatment with the tumor necrosis factor alpha (TNFalpha) antagonist etanercept. In naive mice, exogenous TNFalpha evoked heat hyperalgesia in vivo and sensitized nociceptive nerve fibers to heat in vitro. TNFalpha enhanced the expression of the nociceptor-specific heat transducer ion channel transient receptor potential vanilloid 1 (TRPV1) and increased the amplitudes of capsaicin and heat-activated ionic currents via p38/MAP (mitogen-activated protein) kinase and PKC (protein kinase C). Deletion of the tumor necrosis factor receptor type 2 (TNFR2) gene attenuated heat hyperalgesia and prevented TRPV1 upregulation in tumor-bearing mice, whereas TNFR1 gene deletion played a minor role. We propose endogenous TNFalpha as a key player in cancer-related heat hyperalgesia and nociceptor sensitization that generates TRPV1 upregulation and sensitization via TNFR2.
Figures





References
-
- Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52:744–749. - PubMed
-
- Baumgartner SW, Fleischmann RM, Moreland LW, Schiff MH, Markenson J, Whitmore JB. Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol. 2004;31:1532–1537. - PubMed
-
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
-
- Blumer MJ, Gahleitner P, Narzt T, Handl C, Ruthensteiner B. Ribbons of semithin sections: an advanced method with a new type of diamond knife. J Neurosci Methods. 2002;120:11–16. - PubMed
-
- Cain DM, Wacnik PW, Simone DA. Animal models of cancer pain may reveal novel approaches to palliative care. Pain. 2001a;91:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
