Molecular architecture of a miRNA-regulated 3' UTR
- PMID: 18463285
- PMCID: PMC2441980
- DOI: 10.1261/rna.1082708
Molecular architecture of a miRNA-regulated 3' UTR
Abstract
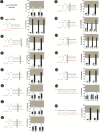
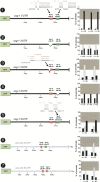
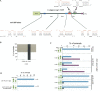
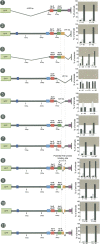
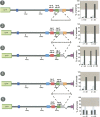
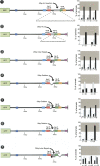
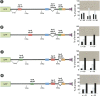
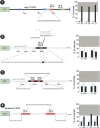
Animal genomes contain hundreds of microRNAs (miRNAs), small regulatory RNAs that control gene expression by binding to complementary sites in target mRNAs. Some rules that govern miRNA/target interaction have been elucidated but their general applicability awaits further experimentation on a case-by-case basis. We use here an assay system in transgenic nematodes to analyze the interaction of the Caenorhabditis elegans lsy-6 miRNA with 3' UTR sequences. In contrast to many previously described assay systems used to analyze miRNA/target interactions, our assay system operates within the cellular context in which lsy-6 normally functions, a single neuron in the nervous system of C. elegans. Through extensive mutational analysis, we define features in the known and experimentally validated target of lsy-6, the 3' UTR of the cog-1 homeobox gene, that are required for a functional miRNA/target interaction. We describe that both in the context of the cog-1 3' UTR and in the context of heterologous 3' UTRs, one or more seed matches are not a reliable predictor for a functional miRNA/target interaction. We rather find that two nonsequence specific contextual features beyond miRNA target sites are critical determinants of miRNA-mediated 3' UTR regulation. The contextual features reside 3' of lsy-6 binding sites in the 3' UTR and act in a combinatorial manner; mutation of each results in limited defects in 3' UTR regulation, but a combinatorial deletion results in complete loss of 3' UTR regulation. Together with two lsy-6 sites, these two contextual features are capable of imparting regulation on a heterologous 3' UTR. Moreover, the contextual features need to be present in a specific configuration relative to miRNA binding sites and could either represent protein binding sites or provide an appropriate structural context. We conclude that a given target site resides in a 3' UTR context that evolved beyond target site complementarity to support regulation by a specific miRNA. The large number of 3' UTRs that we analyzed in this study will also be useful to computational biologists in designing the next generation of miRNA/target prediction algorithms.
Figures




References
-
- Bartel D.P. MicroRNAs. Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Beitzinger M., Peters L., Zhu J.Y., Kremmer E., Meister G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 2007;4:76–84. - PubMed
-
- Bentwich I., Avniel A., Karov Y., Aharonov R., Gilad S., Barad O., Barzilai A., Einat P., Einav U., Meiri E., et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005;37:766–770. - PubMed
-
- Bushati N., Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
