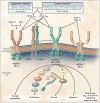
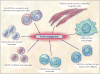
Tumor angiogenesis
- PMID: 18463380
- PMCID: PMC4542009
- DOI: 10.1056/NEJMra0706596
Tumor angiogenesis
Figures

Comment in
-
Tumor angiogenesis.N Engl J Med. 2008 Aug 14;359(7):763; author reply 764. doi: 10.1056/NEJMc081278. N Engl J Med. 2008. PMID: 18703484 No abstract available.
References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. - PubMed
-
- Idem. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. - PubMed
-
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–50. Erratum, N Engl J Med 2007;356:318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
