Prodrugs for the treatment of neglected diseases
- PMID: 18463559
- PMCID: PMC6245083
- DOI: 10.3390/molecules13030616
Prodrugs for the treatment of neglected diseases
Abstract
Recently, World Health Organization (WHO) and Medicins San Frontieres (MSF) proposed a classification of diseases as global, neglected and extremely neglected. Global diseases, such as cancer, cardiovascular and mental (CNS) diseases represent the targets of the majority of the R&D efforts of pharmaceutical companies. Neglected diseases affect millions of people in the world yet existing drug therapy is limited and often inappropriate. Furthermore, extremely neglected diseases affect people living under miserable conditions who barely have access to the bare necessities for survival. Most of these diseases are excluded from the goals of the R&D programs in the pharmaceutical industry and therefore fall outside the pharmaceutical market. About 14 million people,mainly in developing countries, die each year from infectious diseases. From 1975 to 1999,1393 new drugs were approved yet only 1% were for the treatment of neglected diseases[3]. These numbers have not changed until now, so in those countries there is an urgent need for the design and synthesis of new drugs and in this area the prodrug approach is a very interesting field. It provides, among other effects, activity improvements and toxicity decreases for current and new drugs, improving market availability. It is worth noting that it is essential in drug design to save time and money, and prodrug approaches can be considered of high interest in this respect. The present review covers 20 years of research on the design of prodrugs for the treatment of neglected and extremely neglected diseases such as Chagas' disease (American trypanosomiasis), sleeping sickness (African trypanosomiasis), malaria, sickle cell disease, tuberculosis, leishmaniasis and schistosomiasis.
Figures

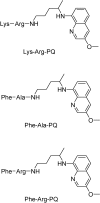

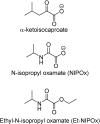
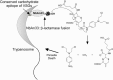

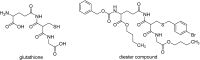
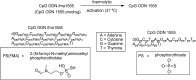




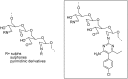
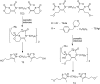


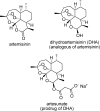

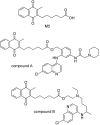

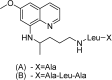
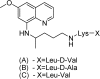

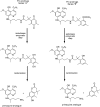
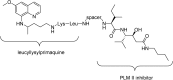

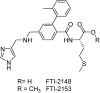
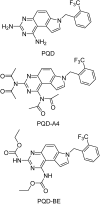
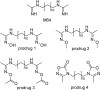

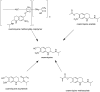

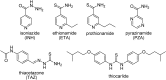

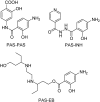
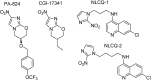
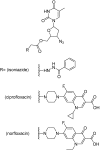
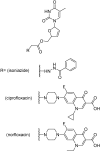
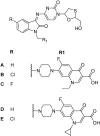




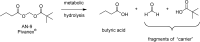
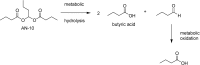
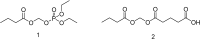
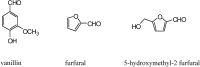
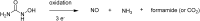
References
-
- Medecins Sans Frontieres Drugs for Neglected Diseases Initiative: Teaming up to address neglect. Mar 12, 2003. [in Nov. 2007]. Accessed from: www.accessmed.msf.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

