Prodrugs for amines
- PMID: 18463563
- PMCID: PMC6245426
- DOI: 10.3390/molecules13030519
Prodrugs for amines
Abstract
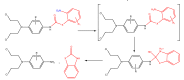
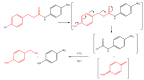
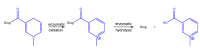
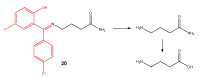
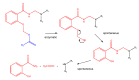
The purpose of this work is to review the published strategies for the production of prodrugs of amines. The review is divided in two main groups of approaches: those that rely on enzymatic activation and those that take advantage of physiological chemical conditions for release of the drugs. A compilation of the most important approaches is presented in the form of a table, where the main advantages and disadvantages of each strategy are also referred.
Figures


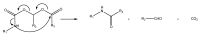
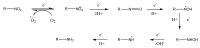
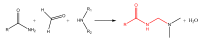

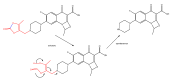

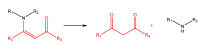
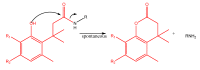

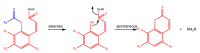
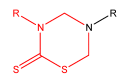
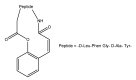
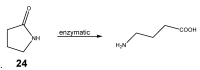
References
-
- Bundgaard H., Johansen M. Prodrugs as drug delivery systems. XIX. Bioreversible derivatisation of aromatic amines by formation of N-Mannich bases with succinimide. Int. J. Pharm. 1981;8:183–192. doi: 10.1016/0378-5173(81)90096-X. - DOI
-
- Bundgaard H. Prodrugs as a means to improve the delivery of peptide drugs. 1. Adv. Drug Deliv. Rev. 1992;8:1–38. doi: 10.1016/0169-409X(92)90014-H. - DOI
-
- Wang W., Jiang J., Ballard C., Wang B. Prodrug approaches to the improved delivery of peptide drugs. Curr. Pharm. Des. 1999;5:265–287. - PubMed
-
- Gasparro D.M., Almeida D.R.P., Pisterzi L.F., Juhasz J.R., Viskolcz B., Penke B., Csizmadia I.G. Reaction profiling of the MAO-B catalyzed oxidative deamination of amines in Alzheimer's disease. J. Mol. Struct.: THEOCHEM. 2003;666-667:527–536. doi: 10.1016/j.theochem.2003.08.077. - DOI
-
- Testa B., Mayer J.M. Hydrolysis in Drug and Prodrug metabolism, Chemistry, biochemistry and enzymology. Wiley-VCH; Weinheim: 2003. p. 83. Chapter 4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

