A structure-activity study of antibacterial diterpenoids
- PMID: 18463590
- PMCID: PMC6245368
- DOI: 10.3390/molecules13040822
A structure-activity study of antibacterial diterpenoids
Abstract
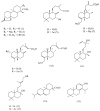
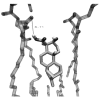
An analysis of the antibacterial activities of 15 terpenoids, eleven of which were previously described by us and four were extracted from the literature, suggested two structural requirements for activity of these and related compounds: a hydrophobic moiety,consisting of a substituted decalin skeleton, and a hydrophilic region possessing one hydrogen-bond-donor group. These structural requirements are responsible for an optimal insertion of these and related compounds into cell membranes, as suggested by the results of docking some of these compounds into a model phospholipid bilayer.
Figures


References
-
- Bankova V., Marcucci M.C., Simova S., Nikolova N., Ujumgiev A., Popova M. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. [C] 1996;51:277–280. - PubMed
-
- Velikova M., Bankova V., Marcucci M.C., Tsvetkova I., Kujumgiev A. Chemical composition and biological activity of propolis from Brazilian meliponinae. Z. Naturforsch. [C] 2000b;55:785–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

