Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient
- PMID: 18463638
- PMCID: PMC2724008
- DOI: 10.1038/nature06923
Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient
Abstract
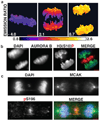
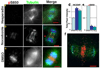
Proper partitioning of the contents of a cell between two daughters requires integration of spatial and temporal cues. The anaphase array of microtubules that self-organize at the spindle midzone contributes to positioning the cell-division plane midway between the segregating chromosomes. How this signalling occurs over length scales of micrometres, from the midzone to the cell cortex, is not known. Here we examine the anaphase dynamics of protein phosphorylation by aurora B kinase, a key mitotic regulator, using fluorescence resonance energy transfer (FRET)-based sensors in living HeLa cells and immunofluorescence of native aurora B substrates. Quantitative analysis of phosphorylation dynamics, using chromosome- and centromere-targeted sensors, reveals that changes are due primarily to position along the division axis rather than time. These dynamics result in the formation of a spatial phosphorylation gradient early in anaphase that is centred at the spindle midzone. This gradient depends on aurora B targeting to a subpopulation of microtubules that activate it. Aurora kinase activity organizes the targeted microtubules to generate a structure-based feedback loop. We propose that feedback between aurora B kinase activation and midzone microtubules generates a gradient of post-translational marks that provides spatial information for events in anaphase and cytokinesis.
Figures


References
-
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. - PubMed
-
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. *9- - PubMed
-
- Rappaport R. Cytokinesis in Animal Cells. Cambridge: Cambridge University Press; 1996.
-
- Alsop GB, Zhang D. Microtubules continuously dictate distribution of actin filaments and positioning of cell cleavage in grasshopper spermatocytes. J Cell Sci. 2004;117:1591–1602. - PubMed
Methods References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

