RAC activity in keloid disease: comparative analysis of fibroblasts from margin of keloid to its surrounding normal skin
- PMID: 18463712
- PMCID: PMC2311454
RAC activity in keloid disease: comparative analysis of fibroblasts from margin of keloid to its surrounding normal skin
Abstract
Background: Keloids are characterized by excess collagen deposition within the dermis. Although the exact cause of the potentially overactive fibroblasts has yet to be elucidated, many etiological possibilities have been suggested. As fibroblasts originating from keloids appear to have an increased migration and proliferation rate, cell-signaling studies examining these factors may offer an opportunity to further our understanding of the pathogenesis of this disease. One of such cell-signaling messengers is the enzyme Ras-related C3 botulinum toxin substrate (RAC), which has never been investigated in keloid scars.
Objective: This study explores the role of RAC activity in keloid disease.
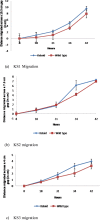
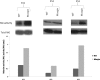
Method: Primary fibroblast cell lines were established from the margin of keloid (KF) scars as well as from the surrounding normal tissue (NF) from one anatomical site of the same patient. Migration and proliferation assays were performed, comparing matching NFs and KFs, and after cell lysis, RAC activity was assessed.
Results: Comparing fibroblasts from 3 different patients, KFs migrated (P < .05) and proliferated (P < .05) faster than NFs. The activity levels of RAC were increased in KFs compared with NFs.
Conclusion: KFs migrate and proliferate faster than NFs. RAC activity increases in KFs when compared with NFs. Inhibition of RAC could lead to a new therapeutic approach.
Figures


Similar articles
-
Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts.Int J Mol Sci. 2017 May 12;18(5):1044. doi: 10.3390/ijms18051044. Int J Mol Sci. 2017. PMID: 28498357 Free PMC article.
-
Increased Cthrc1 Activates Normal Fibroblasts and Suppresses Keloid Fibroblasts by Inhibiting TGF-β/Smad Signal Pathway and Modulating YAP Subcellular Location.Curr Med Sci. 2018 Oct;38(5):894-902. doi: 10.1007/s11596-018-1959-1. Epub 2018 Oct 20. Curr Med Sci. 2018. PMID: 30341526
-
Multitranscriptome analyses of keloid fibroblasts reveal the role of the HIF-1α/HOXC6/ERK axis in keloid development.Burns Trauma. 2022 May 9;10:tkac013. doi: 10.1093/burnst/tkac013. eCollection 2022. Burns Trauma. 2022. PMID: 35547861 Free PMC article.
-
Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets.Int J Mol Sci. 2024 Jan 19;25(2):1235. doi: 10.3390/ijms25021235. Int J Mol Sci. 2024. PMID: 38279232 Free PMC article. Review.
-
The Cellular Response of Keloids and Hypertrophic Scars to Botulinum Toxin A: A Comprehensive Literature Review.Dermatol Surg. 2018 Feb;44(2):149-157. doi: 10.1097/DSS.0000000000001360. Dermatol Surg. 2018. PMID: 29401161 Review.
Cited by
-
Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts.Int J Mol Sci. 2017 May 12;18(5):1044. doi: 10.3390/ijms18051044. Int J Mol Sci. 2017. PMID: 28498357 Free PMC article.
-
Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids.Am J Pathol. 2011 Oct;179(4):1951-60. doi: 10.1016/j.ajpath.2011.06.034. Epub 2011 Aug 26. Am J Pathol. 2011. PMID: 21872564 Free PMC article.
-
Angiotensin-II mediates nonmuscle myosin II activation and expression and contributes to human keloid disease progression.Mol Med. 2011;17(11-12):1196-203. doi: 10.2119/molmed.2010.00265. Epub 2011 Jul 21. Mol Med. 2011. PMID: 21792479 Free PMC article.
-
The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models.Front Cell Dev Biol. 2020 May 26;8:360. doi: 10.3389/fcell.2020.00360. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32528951 Free PMC article. Review.
-
Downregulation of CR6-interacting factor 1 suppresses keloid fibroblast growth via the TGF-β/Smad signaling pathway.Sci Rep. 2021 Jan 12;11(1):500. doi: 10.1038/s41598-020-79785-y. Sci Rep. 2021. PMID: 33436666 Free PMC article.
References
-
- TS Alster, EL Tanzi. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4(4):235–43. - PubMed
-
- J Jagadeesan, A Bayat. Transforming growth factor beta (TGFbeta) and keloid disease. Int J Surg. 2007;5(4):278–85. - PubMed
-
- AS Prado, M Fontbona. A 1.8 kg keloid on the arm. Plast Reconstr Surg. 2006;117:335–36. - PubMed
-
- L Satish, J Lyons-Weiler, PA Hebda, et al. Gene expression patterns in isolated keloid fibroblasts. Wound Rep Regen. 2006;16:463–70. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
