Rab proteins and Rab-associated proteins: major actors in the mechanism of protein-trafficking disorders
- PMID: 18463892
- PMCID: PMC2413085
- DOI: 10.1007/s00431-008-0740-z
Rab proteins and Rab-associated proteins: major actors in the mechanism of protein-trafficking disorders
Abstract
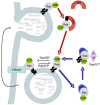
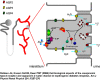
Ras-associated binding (Rab) proteins and Rab-associated proteins are key regulators of vesicle transport, which is essential for the delivery of proteins to specific intracellular locations. More than 60 human Rab proteins have been identified, and their function has been shown to depend on their interaction with different Rab-associated proteins regulating Rab activation, post-translational modification and intracellular localization. The number of known inherited disorders of vesicle trafficking due to Rab cycle defects has increased substantially during the past decade. This review describes the important role played by Rab proteins in a number of rare monogenic diseases as well as common multifactorial human ones. Although the clinical phenotype in these monogenic inherited diseases is highly variable and dependent on the type of tissue in which the defective Rab or its associated protein is expressed, frequent features are hypopigmentation (Griscelli syndrome), eye defects (Choroideremia, Warburg Micro syndrome and Martsolf syndrome), disturbed immune function (Griscelli syndrome and Charcot-Marie-Tooth disease) and neurological dysfunction (X-linked non-specific mental retardation, Charcot-Marie-Tooth disease, Warburg Micro syndrome and Martsolf syndrome). There is also evidence that alterations in Rab function play an important role in the progression of multifactorial human diseases, such as infectious diseases and type 2 diabetes. Rab proteins must not only be bound to GTP, but they need also to be 'prenylated'-i.e. bound to the cell membranes by isoprenes, which are intermediaries in the synthesis of cholesterol (e.g. geranyl geranyl or farnesyl compounds). This means that isoprenylation can be influenced by drugs such as statins, which inhibit isoprenylation, or biphosphonates, which inhibit that farnesyl pyrophosphate synthase necessary for Rab GTPase activity.
Conclusion: Although protein-trafficking disorders are clinically heterogeneous and represented in almost every subspeciality of pediatrics, the identification of common pathogenic mechanisms may provide a better diagnosis and management of patients with still unknown Rab cycle defects and stimulate the development of therapeutic agents.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/sj.emboj.7601008', 'is_inner': False, 'url': 'https://doi.org/10.1038/sj.emboj.7601008'}, {'type': 'PMC', 'value': 'PMC1409716', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1409716/'}, {'type': 'PubMed', 'value': '16498408', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16498408/'}]}
- Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R (2006) ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis. EMBO J 25:977–986 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC546431', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC546431/'}, {'type': 'PubMed', 'value': '15690078', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15690078/'}]}
- Aizawa T, Komatsu M (2005) Rab27a: a new face in beta cell metabolism-secretion coupling. J Clin Invest 115:227–230 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC395482', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC395482/'}, {'type': 'PubMed', 'value': '7957092', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7957092/'}]}
- Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra MC, Zerial M (1994) Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated Rab proteins to their target membranes. EMBO J 13:5262−5273 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/ng1517', 'is_inner': False, 'url': 'https://doi.org/10.1038/ng1517'}, {'type': 'PubMed', 'value': '15696165', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15696165/'}]}
- Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina EN, Morgan NV, Tee L, Morton J, Ainsworth JR, Horn D, Rosser E, Cole TR, Stolte-Dijkstra I, Fieggen K, Clayton-Smith J, Mégarbané A, Shield JP, Newbury-Ecob R, Dobyns WB, Graham JM Jr, Kjaer KW, Warburg M, Bond J, Trembath RC, Harris LW, Takai Y, Mundlos S, Tannahill D, Woods CG, Maher ER (2005) Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet 37:221–223 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1086/502681', 'is_inner': False, 'url': 'https://doi.org/10.1086/502681'}, {'type': 'PMC', 'value': 'PMC1424696', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1424696/'}, {'type': 'PubMed', 'value': '16532399', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16532399/'}]}
- Aligianis IA, Morgan NV, Mione M, Johnson CA, Rosser E, Hennekam RC, Adams G, Trembath RC, Pilz DT, Stoodley N, Moore AT, Wilson S, Maher ER (2006) Mutation in Rab3 GTPase-activating protein (RAB3GAP) noncatalytic subunit in a kindred with Martsolf syndrome. Am J Hum Genet 78:702–707 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

