Mapping the signal-to-noise-ratios of cortical sources in magnetoencephalography and electroencephalography
- PMID: 18465745
- PMCID: PMC2882168
- DOI: 10.1002/hbm.20571
Mapping the signal-to-noise-ratios of cortical sources in magnetoencephalography and electroencephalography
Abstract
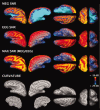
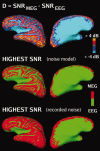
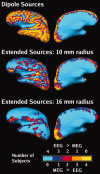
Although magnetoencephalography (MEG) and electroencephalography (EEG) have been available for decades, their relative merits are still debated. We examined regional differences in signal-to-noise-ratios (SNRs) of cortical sources in MEG and EEG. Data from four subjects were used to simulate focal and extended sources located on the cortical surface reconstructed from high-resolution magnetic resonance images. The SNR maps for MEG and EEG were found to be complementary. The SNR of deep sources was larger in EEG than in MEG, whereas the opposite was typically the case for superficial sources. Overall, the SNR maps were more uniform for EEG than for MEG. When using a noise model based on uniformly distributed random sources on the cortex, the SNR in MEG was found to be underestimated, compared with the maps obtained with noise estimated from actual recorded MEG and EEG data. With extended sources, the total area of cortex in which the SNR was higher in EEG than in MEG was larger than with focal sources. Clinically, SNR maps in a patient explained differential sensitivity of MEG and EEG in detecting epileptic activity. Our results emphasize the benefits of recording MEG and EEG simultaneously.
2008 Wiley-Liss, Inc.
Figures

References
-
- Ahonen AI,Hämäläinen MS,Ilmoniemi RJ,Kajola MJ,Knuutila JE,Simola JT,Vilkman VA( 1993): Sampling theory for neuromagnetic detector arrays. IEEE Trans Biomed Eng 40: 859–869. - PubMed
-
- Barkley GL,Baumgartner C( 2003): MEG and EEG in epilepsy. J Clin Neurophysiol 20: 163–178. - PubMed
-
- Baumgartner C( 2004): Controversies in clinical neurophysiology. MEG is superior to EEG in the localization of interictal epileptiform activity: Con. Clin Neurophysiol 115: 1010–1020. - PubMed
-
- Baumgartner C,Pataraia E,Lindinger G,Deecke L( 2000): Neuromagnetic recordings in temporal lobe epilepsy. J Clin Neurophysiol 17: 177–189. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

