A new technique for primary hepatocyte expansion in vitro
- PMID: 18465801
- PMCID: PMC4487520
- DOI: 10.1002/bit.21911
A new technique for primary hepatocyte expansion in vitro
Abstract
The current application for many potential cell-based treatments for liver failure is limited by the low availability of mature functional hepatocytes. Although adult hepatocytes have a remarkable ability to proliferate in vivo, attempts to proliferate adult hepatocytes in vitro have been less successful. In this study, we investigated the effect of coculture cell type on the proliferative response and the functional activities of hepatocytes. We show, for the first time, a robust proliferative response of primary adult rat hepatocytes when cocultured with mouse 3T3-J2 fibroblasts. Hepatocytes cultured at low density on growth-arrested 3T3-J2 fibroblast feeder layers underwent significantly higher proliferation rates than when cultured on feeder layers made of four other cell types. Increasing colony size correlated with an increase in hepatocellular functions. The proliferating hepatocytes retained their morphologic, phenotypic, and functional characteristics. Using a cell patterning technique, we found that 3T3-J2 fibroblasts stimulate DNA synthesis in hepatocytes by short-range heterotypic cell-cell interactions. When hepatocytes that proliferated in cocultures were harvested and further subcultured either on 3T3-J2 fibroblast feeders or in the collagen sandwich configuration, their behavior was similar to that of freshly isolated hepatocytes. We conclude that adult rat hepatocytes can proliferate in vitro in a coculture cell type-dependent manner, and can be serially propagated by coculturing with 3T3-J2 fibroblasts while maintaining their differentiated characteristics. Our results also suggest that one of the major reasons for the functional differences in hepatocyte cocultures may be due to the different proliferative responses of hepatocytes as a function of coculture cell type. This study provides new insights in the roles of coculture cell types and cell-cell interactions in the modulation of hepatic proliferation and function.
Figures





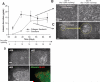
References
-
- Asahina K, Fujimori H, Shimizu-Saito K, Kumashiro Y, Okamura K, Tanaka Y, Teramoto K, Arii S, Teraoka H. Expression of the liver-specific gene Cyp7a1 reveals hepatic differentiation in embryoid bodies derived from mouse embryonic stem cells. Genes Cells. 2004;9(12):1297–1308. - PubMed
-
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: Hepatocyte function in microfabricated co-cultures. J Biomater Sci Polym Ed. 1998;9(11):1137–1160. - PubMed
-
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. - PubMed
-
- Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132(6):1133–1149. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

