SMAD3 functions as a transcriptional repressor of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc
- PMID: 18466073
- PMCID: PMC2684157
- DOI: 10.1359/jbmr.080502
SMAD3 functions as a transcriptional repressor of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc
Abstract
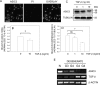
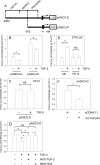
The goal of this investigation was to study the regulation of acid-sensing ion channel (ASIC)3 expression by TGFbeta in the nucleus pulposus cells of the intervertebral disc. Analysis of human nucleus pulposus tissue indicated decreased ASIC3 and elevated TGFbeta expression in the degenerate state. In a parallel study, treatment of nucleus pulposus cells with TGFbeta resulted in decreased expression of ASIC3 mRNA and protein. Suppression of ASIC3 promoter activity was evident when the nucleus pulposus cells were treated with TGFbeta or co-transfected with the constitutively active ALK5 or a smad3 construct. On the other hand, co-transfection of dominant negative smad3 or smad7 restored ASIC3 promoter activity. We validated the role of smad3 in controlling ASIC3 expression using cells derived from smad3-null mice. ASIC3 promoter activity in the null cells was 2- to 3-fold higher than the wildtype cells. Moreover, expression of smad3 in null cells decreased ASIC3 promoter activity by almost 50%. Further studies using deletion constructs and trichostatin A treatment showed that the full-length smad3 was necessary, and the suppression involved recruitment of histone deacetylase to the promoter. To determine the mechanism, we evaluated the rat ASIC3 promoter sequence and noted the presence of two smad interacting CAGA box motifs. Gel-shift and supershift analysis indicated that smad3 protein was bound to this motif. Chromatin immunoprecipitation analysis confirmed that smad3 bound both the CAGA elements. Results of these studies clearly show that TGFbeta is highly expressed in the degenerate disc and through smad3 serves as a negative regulator of ASIC3 expression.
Figures




References
-
- Hassler O. The human intervertebral disc. A micro-angiographical study on its vascular supply at various ages. Acta Orthop Scand. 1969;40:765–772. - PubMed
-
- Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. - PubMed
-
- Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud M. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–C631. - PubMed
-
- Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88:30–35. - PubMed
-
- Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. - PubMed

