Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection
- PMID: 18466298
- PMCID: PMC2615191
- DOI: 10.1111/j.1365-2958.2008.06271.x
Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection
Abstract
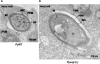
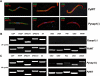
Malaria parasite sporozoites prepare for transmission to a mammalian host by upregulation of UIS (Upregulated in Infectious Sporozoites) genes. A number of UIS gene products are essential for the establishment of the intrahepatocytic niche. However, the factors that regulate the expression of genes involved in gain of infectivity for the liver are unknown. Herein, we show that a conserved Plasmodium sporozoite low-complexity asparagine-rich protein, SAP1 (Sporozoite Asparagine-rich Protein 1), has an essential role in malaria parasite liver infection. Targeted deletion of SAP1 in the rodent malaria parasite Plasmodium yoelii generated mutant parasites that traverse and invade hepatocytes normally but cannot initiate liver-stage development in vitro and in vivo. Moreover, immunizations with Pysap1(-) sporozoites confer long-lasting sterile protection against wild-type sporozoite infection. Strikingly, lack of SAP1 abolished expression of essential UIS genes including UIS3, UIS4 and P52 but not the constitutively expressed genes encoding, among others, sporozoite proteins CSP and TRAP. SAP1 localization to the cell interior but not the nucleus of sporozoites suggests its involvement in a post-transcriptional mechanism of gene expression control. These findings demonstrate that SAP1 is essential for liver infection possibly by functioning as a selective regulator controlling the expression of infectivity-associated parasite effector genes.
Figures




References
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. - PubMed
-
- Aravind L, Iyer LM, Wellems TE, Miller LH. Plasmodium biology: genomic gleanings. Cell. 2003;115:771–785. - PubMed
-
- Baldacci P, Menard R. The elusive malaria sporozoite in the mammalian host. Mol Microbiol. 2004;54:298–306. - PubMed
-
- Belmonte M, Jones TR, Lu M, Arcilla R, Smalls T, Belmonte A, et al. The infectivity of Plasmodium yoelii in different strains of mice. J Parasitol. 2003;89:602–603. - PubMed
-
- Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, et al. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci. 2003;116:39–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

