Discontinuation rates in clinical trials in musculoskeletal pain: meta-analysis from etoricoxib clinical trial reports
- PMID: 18466615
- PMCID: PMC2483442
- DOI: 10.1186/ar2422
Discontinuation rates in clinical trials in musculoskeletal pain: meta-analysis from etoricoxib clinical trial reports
Abstract
Introduction: Patient adherence to therapy in clinical practice is often low, and the difference between efficacy measured in clinical trials and effectiveness in clinical practice is probably a function of discontinuation of therapy because of lack of efficacy or because of unmanageable or intolerable adverse events. Discontinuation is frequently measured in clinical trials but is not usually described in detail in published reports, often because of limitations in the size of publications. By contrast, company clinical trial reports include much more detail.
Methods: We examined company clinical trial reports of trials involving etoricoxib in four musculoskeletal conditions: osteoarthritis, rheumatoid arthritis, chronic low back pain and ankylosing spondylitis. Information was available from 18 randomized trials (10,143 patients) lasting 4 to 12 weeks (one 4 weeks, three 6 weeks, one 8 weeks and seven 12 weeks) and from three trials with a mean duration of about 80 weeks (34,695 patients). These clinical trial reports contain over 73,000 pages of information.
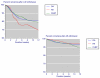
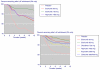
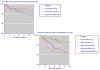
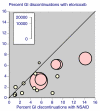
Results: Over 12 weeks, lack of efficacy and adverse event discontinuations were similar between osteoarthritis, rheumatoid arthritis and back pain, with lack of efficacy discontinuation rates some three times higher than for adverse events. All-cause and lack of efficacy discontinuations were lower with etoricoxib (all doses combined) and traditional nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) than with placebo, although NSAIDs produced higher rates of clinical adverse events and gastrointestinal discontinuations than did placebo. Etoricoxib had fewer discontinuations than NSAIDs for lack of efficacy, clinical adverse events, and laboratory and gastrointestinal adverse events, but with more discontinuations because of hypertension and oedema. Comparison with two similar meta-analyses of other cyclo-oxygenase-2 selective inhibitors (more than 80,000 patients in total) revealed consistency between analyses.
Conclusion: Examining discontinuation data from clinical trials, even when the numbers of patients are very large, does not necessarily predict what will happen in the real world, where clinical effectiveness may differ from clinical efficacy assessed in trials. Data from these analyses appears to agree with findings from real world practice.
Figures
References
-
- Adult Treatment Panel III Third report on National Cholesterol Education Program expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report: IX adherence. Circulation. 2002;106:3359–3366. http://circ.ahajournals.org/cgi/content/full/106/25/3359?etoc - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

