In vitro hemodynamic investigation of the embryonic aortic arch at late gestation
- PMID: 18466908
- PMCID: PMC3805112
- DOI: 10.1016/j.jbiomech.2008.03.013
In vitro hemodynamic investigation of the embryonic aortic arch at late gestation
Abstract
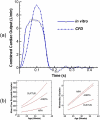
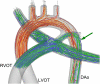
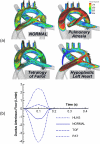
This study focuses on the dynamic flow through the fetal aortic arch driven by the concurrent action of right and left ventricles. We created a parametric pulsatile computational fluid dynamics (CFD) model of the fetal aortic junction with physiologic vessel geometries. To gain a better biophysical understanding, an in vitro experimental fetal flow loop for flow visualization was constructed for identical CFD conditions. CFD and in vitro experimental results were comparable. Swirling flow during the acceleration phase of the cardiac cycle and unidirectional flow following mid-deceleration phase were observed in pulmonary arteries (PA), head-neck vessels, and descending aorta. Right-to-left (oxygenated) blood flowed through the ductus arteriosus (DA) posterior relative to the antegrade left ventricular outflow tract (LVOT) stream and resembled jet flow. LVOT and right ventricular outflow tract flow mixing had not completed until approximately 3.5 descending aorta diameters downstream of the DA insertion into the aortic arch. Normal arch model flow patterns were then compared to flow patterns of four common congenital heart malformations that include aortic arch anomalies. Weak oscillatory reversing flow through the DA junction was observed only for the Tetralogy of Fallot configuration. PA and hypoplastic left heart syndrome configurations demonstrated complex, abnormal flow patterns in the PAs and head-neck vessels. Aortic coarctation resulted in large-scale recirculating flow in the aortic arch proximal to the DA. Intravascular flow patterns spatially correlated with abnormal vascular structures consistent with the paradigm that abnormal intravascular flow patterns associated with congenital heart disease influence vascular growth and function.
Figures




Similar articles
-
Effects of pulmonary artery banding and retrograde aortic arch obstruction on the hybrid palliation of hypoplastic left heart syndrome.J Thorac Cardiovasc Surg. 2013 Dec;146(6):1341-8. doi: 10.1016/j.jtcvs.2013.01.038. Epub 2013 Feb 20. J Thorac Cardiovasc Surg. 2013. PMID: 23434295
-
Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass.J Biomech Eng. 2008 Dec;130(6):061012. doi: 10.1115/1.2978988. J Biomech Eng. 2008. PMID: 19045541
-
Experimental and computational study of pulsatile flow characteristics in Romanesque and gothic aortic arch models.Med Eng Phys. 2022 Apr;102:103784. doi: 10.1016/j.medengphy.2022.103784. Epub 2022 Feb 25. Med Eng Phys. 2022. PMID: 35346437
-
Congenital obstructive lesions of the right aortic arch.Ann Thorac Surg. 1999 Apr;67(4):1194-202. doi: 10.1016/s0003-4975(98)01325-3. Ann Thorac Surg. 1999. PMID: 10320289 Review.
-
Fetal hemodynamics.J Perinat Med. 2001;29(5):371-80. doi: 10.1515/JPM.2001.053. J Perinat Med. 2001. PMID: 11723838 Review.
Cited by
-
Tetralogy of Fallot Surgical Repair: Shunt Configurations, Ductus Arteriosus and the Circle of Willis.Cardiovasc Eng Technol. 2017 Jun;8(2):107-119. doi: 10.1007/s13239-017-0302-5. Epub 2017 Apr 5. Cardiovasc Eng Technol. 2017. PMID: 28382440 Free PMC article.
-
Tunable Blood Shunt for Neonates With Complex Congenital Heart Defects.Front Bioeng Biotechnol. 2022 Jan 13;9:734310. doi: 10.3389/fbioe.2021.734310. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35096785 Free PMC article.
-
Pilot study of minimum occlusive force of vascular clamps on arterial vessels in rats.Sci Rep. 2021 Mar 15;11(1):6042. doi: 10.1038/s41598-021-84346-y. Sci Rep. 2021. PMID: 33723269 Free PMC article.
-
Cannulation strategy for aortic arch reconstruction using deep hypothermic circulatory arrest.Ann Thorac Surg. 2012 Aug;94(2):614-20. doi: 10.1016/j.athoracsur.2012.03.053. Epub 2012 May 18. Ann Thorac Surg. 2012. PMID: 22608717 Free PMC article.
-
4D subject-specific inverse modeling of the chick embryonic heart outflow tract hemodynamics.Biomech Model Mechanobiol. 2016 Jun;15(3):723-43. doi: 10.1007/s10237-015-0720-y. Epub 2015 Sep 11. Biomech Model Mechanobiol. 2016. PMID: 26361767 Free PMC article.
References
-
- Achiron R, Zimand S, Hegesh J, Lipitz S, Zalel Y, Rotstein Z. Fetal aortic arch measurements between 14 and 38 weeks' gestation: in-utero ultrasonographic study. Ultrasound Obstet Gynecol. 2000;15:226–230. - PubMed
-
- Allan L, Hornberger L, Sharland G. Textbook of Fetal Cardiology. Greenwich Medical Media Limited; London: 2000.
-
- Axt-Fliedner R, Kreiselmaier P, Schwarze A, Krapp M, Gembruch U. Development of hypoplastic left heart syndrome after diagnosis of aortic stenosis in the first trimester by early echocardiography. Ultrasound Obstet Gynecol. 2006;28:106–109. - PubMed
-
- Blackburn S. Placental, fetal, and transitional circulation revisited. J Perinat Neonatal Nurs. 2006;20:290–294. - PubMed
-
- Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, Kachaner J, Sidi D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

