Functional circularity of legitimate Qbeta replicase templates
- PMID: 18466922
- PMCID: PMC7173182
- DOI: 10.1016/j.jmb.2008.03.074
Functional circularity of legitimate Qbeta replicase templates
Abstract
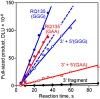
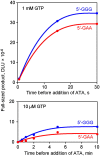
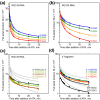
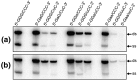
Qbeta replicase (RNA-directed RNA polymerase of bacteriophage Qbeta) exponentially amplifies certain RNAs in vitro. Previous studies have shown that Qbeta replicase can initiate and elongate on a variety of RNAs; however, only a minute fraction of them are recognized as 'legitimate' templates. Guanosine 5'-triphosphate (GTP)-dependent initiation on a legitimate template generates a stable replicative complex capable of elongation in the presence of aurintricarboxylic acid, a powerful inhibitor of RNA-protein interactions. On the contrary, initiation on an illegitimate template is GTP independent and does not result in the aurintricarboxylic-acid-resistant replicative complex. This article demonstrates that the 3' and 5' termini of a legitimate template cooperate during and after the initiation step. Breach of the cooperation by dividing the template into fragments or by introducing point mutations at the 5' terminus reduces the rate and the yield of initiation, increases the GTP requirement, decreases the overall rate of template copying, and destabilizes the postinitiation replicative complex. These results revive the old idea of a functional circularity of legitimate Qbeta replicase templates and complement the increasing body of evidence that functional circularity may be a common property of RNA templates directing the synthesis of either RNA or protein molecules.
Figures


Similar articles
-
Qbeta replicase discriminates between legitimate and illegitimate templates by having different mechanisms of initiation.J Biol Chem. 2003 Nov 7;278(45):44139-46. doi: 10.1074/jbc.M305992200. Epub 2003 Aug 27. J Biol Chem. 2003. PMID: 12947121
-
Probing the legitimate initiation of RNA synthesis by Qβ replicase with oligonucleotide primers.FEBS Lett. 2024 Mar;598(5):579-586. doi: 10.1002/1873-3468.14833. Epub 2024 Feb 26. FEBS Lett. 2024. PMID: 38408766
-
Q beta replicase template specificity: different templates require different GTP concentrations for initiation.Proc Natl Acad Sci U S A. 1980 May;77(5):2601-5. doi: 10.1073/pnas.77.5.2601. Proc Natl Acad Sci U S A. 1980. PMID: 6930654 Free PMC article.
-
Structures and functions of Qβ replicase: translation factors beyond protein synthesis.Int J Mol Sci. 2014 Sep 2;15(9):15552-70. doi: 10.3390/ijms150915552. Int J Mol Sci. 2014. PMID: 25184952 Free PMC article. Review.
-
[Unsolved Puzzles of Qβ Replicase].Mol Biol (Mosk). 2019 Nov-Dec;53(6):899-910. doi: 10.1134/S0026898419060041. Mol Biol (Mosk). 2019. PMID: 31876271 Review. Russian.
Cited by
-
Structural transition of replicable RNAs during in vitro evolution with Qβ replicase.RNA. 2020 Jan;26(1):83-90. doi: 10.1261/rna.073106.119. Epub 2019 Nov 5. RNA. 2020. PMID: 31690585 Free PMC article.
-
A design principle for a single-stranded RNA genome that replicates with less double-strand formation.Nucleic Acids Res. 2015 Sep 18;43(16):8033-43. doi: 10.1093/nar/gkv742. Epub 2015 Jul 21. Nucleic Acids Res. 2015. PMID: 26202975 Free PMC article.
-
Applications of phage-derived RNA-based technologies in synthetic biology.Synth Syst Biotechnol. 2020 Dec;5(4):343-360. doi: 10.1016/j.synbio.2020.09.003. Epub 2020 Oct 16. Synth Syst Biotechnol. 2020. PMID: 33083579 Free PMC article. Review.
-
Identification of two forms of Q{beta} replicase with different thermal stabilities but identical RNA replication activity.J Biol Chem. 2010 Nov 26;285(48):37210-7. doi: 10.1074/jbc.M110.117846. Epub 2010 Sep 21. J Biol Chem. 2010. PMID: 20858892 Free PMC article.
-
Cell-free expression of RNA encoded genes using MS2 replicase.Nucleic Acids Res. 2019 Nov 18;47(20):10956-10967. doi: 10.1093/nar/gkz817. Nucleic Acids Res. 2019. PMID: 31566241 Free PMC article.
References
-
- Blumenthal T., Carmichael G. RNA replication: function and structure of Qβ replicase. Annu. Rev. Biochem. 1979;48:525–545. - PubMed
-
- van Duin J. Single-stranded RNA bacteriophages. In: Calendar R., editor. vol. 1. Plenum Press; New York: 1988. pp. 117–167. (The Bacteriophages).
-
- Chetverin A.B., Spirin A.S. Replicable RNA vectors: prospects for cell-free gene amplification, expression and cloning. Prog. Nucleic Acid Res. Mol. Biol. 1995;51:225–270. - PubMed
-
- Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. - PubMed
-
- Haruna I., Spiegelman S. Autocatalytic synthesis of a viral RNA in vitro. Science. 1965;150:884–886. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

