Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis
- PMID: 18467368
- PMCID: PMC2538809
- DOI: 10.1113/jphysiol.2007.149286
Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis
Abstract
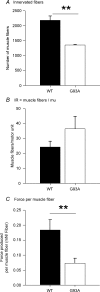
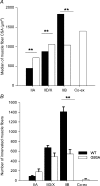
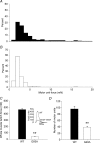
The present study investigated motor unit (MU) loss in a murine model of familial amyotrophic lateral sclerosis (ALS). The fast-twitch tibialis anterior (TA) and medial gastrocnemius (MG) muscles of transgenic SOD1(G93A) and SOD1(WT) mice were studied during the presymptomatic phase of disease progression at 60 days of age. Whole muscle maximum isometric twitch and tetanic forces were 80% lower (P < 0.01) in the TA muscles of SOD1(G93A) compared to SOD1(WT) mice. Enumeration of total MU numbers within TA muscles showed a 60% reduction (P < 0.01) within SOD1(G93A) mice (38 +/- 7) compared with SOD1(WT) controls (95 +/- 12); this was attributed to a lower proportion of the most forceful fast-fatigable (FF) MU in SOD1(G93A) mice, as seen by a significant (P < 0.01) leftward shift in the cumulative frequency histogram of single MU forces. Similar patterns of MU loss and corresponding decreases in isometric twitch force were observed in the MG. Immunocytochemical analyses of the entire cross-sectional area (CSA) of serial sections of TA muscles stained with anti-neural cell adhesion molecule (NCAM) and various monoclonal antibodies for myosin heavy chain (MHC) isoforms showed respective 65% (P < 0.01) and 28% (P < 0.05) decreases in the number of innervated IIB and IID/X muscle fibres in SOD1(G93A), which paralleled the 60% decrease (P < 0.01) in the force generating capacity of individual fibres. The loss of fast MUs was partially compensated by activity-dependent fast-to-slower fibre type transitions, as determined by increases (P < 0.04) in the CSA and proportion of IIA fibres (from 4% to 14%) and IID/X fibres (from 31% to 39%), and decreases (P < 0.001) in the CSA and proportion of type IIB fibres (from 65% to 44%). We conclude that preferential loss of IIB fibres is incomplete at 60 days of age, and is consistent with a selective albeit gradual loss of FF MUs that is not fully compensated by sprouting of the remaining motoneurons that innervate type IIA or IID/X muscle fibres. Our findings indicate that disease progression in fast-twitch muscles of SOD1(G93A) mice involves parallel processes: (1) gradual selective motor axon die-back of the FF motor units that contain large type IIB muscle fibres, and of fatigue-intermediate motor units that innervate type IID/X muscle fibres, and (2) activity-dependent conversion of motor units to those innervated by smaller motor axons innervating type IIA fatigue-resistant muscle fibres.
Figures
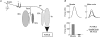





Similar articles
-
Functional over-load saves motor units in the SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis.Neurobiol Dis. 2010 Feb;37(2):412-22. doi: 10.1016/j.nbd.2009.10.021. Epub 2009 Oct 29. Neurobiol Dis. 2010. PMID: 19879358
-
Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis.Neurobiol Dis. 2007 Nov;28(2):154-64. doi: 10.1016/j.nbd.2007.07.003. Epub 2007 Jul 10. Neurobiol Dis. 2007. PMID: 17766128
-
Crush injury to motor nerves in the G93A transgenic mouse model of amyotrophic lateral sclerosis promotes muscle reinnervation and survival of functionally intact nerve-muscle contacts.Neurobiol Dis. 2018 May;113:33-44. doi: 10.1016/j.nbd.2018.01.019. Epub 2018 Jan 31. Neurobiol Dis. 2018. PMID: 29409912
-
Is SOD1 loss of function involved in amyotrophic lateral sclerosis?Brain. 2013 Aug;136(Pt 8):2342-58. doi: 10.1093/brain/awt097. Epub 2013 May 17. Brain. 2013. PMID: 23687121 Free PMC article. Review.
-
Electrical and Morphological Properties of Developing Motoneurons in Postnatal Mice and Early Abnormalities in SOD1 Transgenic Mice.Adv Neurobiol. 2022;28:353-373. doi: 10.1007/978-3-031-07167-6_14. Adv Neurobiol. 2022. PMID: 36066832 Review.
Cited by
-
Skeletal Muscle in ALS: An Unappreciated Therapeutic Opportunity?Cells. 2021 Mar 2;10(3):525. doi: 10.3390/cells10030525. Cells. 2021. PMID: 33801336 Free PMC article. Review.
-
Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motoneurons.J Neurosci. 2010 Apr 21;30(16):5544-58. doi: 10.1523/JNEUROSCI.0434-10.2010. J Neurosci. 2010. PMID: 20410108 Free PMC article.
-
Chemogenetic inhibition of TrkB signalling reduces phrenic motor neuron survival and size.Mol Cell Neurosci. 2023 Jun;125:103847. doi: 10.1016/j.mcn.2023.103847. Epub 2023 Mar 21. Mol Cell Neurosci. 2023. PMID: 36958643 Free PMC article.
-
Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice.PLoS One. 2011;6(9):e24189. doi: 10.1371/journal.pone.0024189. Epub 2011 Sep 1. PLoS One. 2011. PMID: 21909419 Free PMC article.
-
Increased [18F]DPA-714 Uptake in the Skeletal Muscle of SOD1G93A Mice: A New Potential of Translocator Protein 18 kDa Imaging in Amyotrophic Lateral Sclerosis.Biomolecules. 2025 May 31;15(6):799. doi: 10.3390/biom15060799. Biomolecules. 2025. PMID: 40563439 Free PMC article.
References
-
- Atkin JD, Scott RL, West JM, Lopes E, Quah AK, Cheema SS. Properties of slow- and fast-twitch muscle fibres in a mouse model of amyotrophic lateral sclerosis. Neuromuscul Disord. 2005;15:377–388. - PubMed
-
- Azzouz M, Leclerc N, Gurney M, Warter JM, Poindron P, Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:45–51. - PubMed
-
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. - PubMed
-
- Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24:515–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

