Short-term potentiation of mEPSCs requires N-, P/Q- and L-type Ca2+ channels and mitochondria in the supraoptic nucleus
- PMID: 18467369
- PMCID: PMC2538772
- DOI: 10.1113/jphysiol.2007.148957
Short-term potentiation of mEPSCs requires N-, P/Q- and L-type Ca2+ channels and mitochondria in the supraoptic nucleus
Abstract
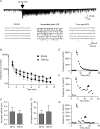
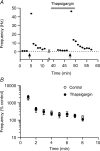
The glutamatergic synapses of the supraoptic nucleus display a unique activity-dependent plasticity characterized by a barrage of tetrodotoxin-resistant miniature EPSCs (mEPSCs) persisting for 5-20 min, causing postsynaptic excitation. We investigated how this short-term synaptic potentiation (STP) induced by a brief high-frequency stimulation (HFS) of afferents was initiated and maintained without lingering presynaptic firing, using in vitro patch-clamp recording on rat brain slices. We found that following the immediate rise in mEPSC frequency, STP decayed with two-exponential functions indicative of two discrete phases. STP depends entirely on extracellular Ca(2+) which enters the presynaptic terminals through voltage-gated Ca(2+) channels but also, to a much lesser degree, through a pathway independent of these channels or reverse mode of the plasma membrane Na(+)-Ca(2+) exchanger. Initiation of STP is largely mediated by any of the N-, P/Q- or L-type channels, and only a simultaneous application of specific blockers for all these channels attenuates STP. Furthermore, the second phase of STP is curtailed by the inhibition of mitochondrial Ca(2+) uptake or mitochondrial Na(+)-Ca(2+) exchanger. mEPSCs amplitude is also potentiated by HFS which requires extracellular Ca(2+). In conclusion, induction of mEPSC-STP is redundantly mediated by presynaptic N-, P/Q- and L-type Ca(2+) channels while the second phase depends on mitochondrial Ca(2+) sequestration and release. Since glutamate influences unique firing patterns that optimize hormone release by supraoptic magnocellular neurons, a prolonged barrage of spontaneous excitatory transmission may aid in the induction of respective firing activities.
Figures






Similar articles
-
Multivesicular release underlies short term synaptic potentiation independent of release probability change in the supraoptic nucleus.PLoS One. 2013 Sep 24;8(9):e77402. doi: 10.1371/journal.pone.0077402. eCollection 2013. PLoS One. 2013. PMID: 24086774 Free PMC article.
-
Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons.J Neurophysiol. 2000 May;83(5):2542-53. doi: 10.1152/jn.2000.83.5.2542. J Neurophysiol. 2000. PMID: 10805656
-
GABA(B) receptors modulate short-term potentiation of spontaneous excitatory postsynaptic currents in the rat supraoptic nucleus in vitro.Neuropharmacology. 2001 Oct;41(5):554-64. doi: 10.1016/s0028-3908(01)00098-3. Neuropharmacology. 2001. PMID: 11587710
-
Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus.Prog Brain Res. 2002;139:235-46. doi: 10.1016/s0079-6123(02)39020-4. Prog Brain Res. 2002. PMID: 12436939 Review.
-
Pre- and postsynaptic modulation of the electrical activity of rat supraoptic neurones.Exp Physiol. 2000 Mar;85 Spec No:145S-151S. doi: 10.1111/j.1469-445x.2000.tb00018.x. Exp Physiol. 2000. PMID: 10795917 Review.
Cited by
-
Acute intermittent hypoxia in neonatal rodent central nervous system facilitates respiratory frequency through the recruitment of hypothalamic areas.Exp Physiol. 2025 Sep;110(9):1358-1376. doi: 10.1113/EP092303. Epub 2025 Mar 13. Exp Physiol. 2025. PMID: 40083110 Free PMC article.
-
Specificity in the interaction of high-voltage-activated Ca2+ channel types with Ca2+-dependent afterhyperpolarizations in magnocellular supraoptic neurons.J Neurophysiol. 2018 Oct 1;120(4):1728-1739. doi: 10.1152/jn.00285.2018. Epub 2018 Jul 18. J Neurophysiol. 2018. PMID: 30020842 Free PMC article.
-
COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca(2+) influx in the subfornical organ.Am J Physiol Heart Circ Physiol. 2013 Nov 15;305(10):H1451-61. doi: 10.1152/ajpheart.00238.2013. Epub 2013 Sep 6. Am J Physiol Heart Circ Physiol. 2013. PMID: 24014678 Free PMC article.
-
Multivesicular release underlies short term synaptic potentiation independent of release probability change in the supraoptic nucleus.PLoS One. 2013 Sep 24;8(9):e77402. doi: 10.1371/journal.pone.0077402. eCollection 2013. PLoS One. 2013. PMID: 24086774 Free PMC article.
References
-
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. - PubMed
-
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

