The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation
- PMID: 18467434
- PMCID: PMC2488207
- DOI: 10.1210/en.2007-1669
The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation
Abstract
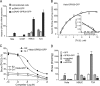
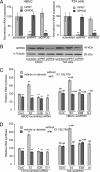
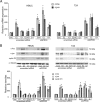
We have previously shown that estrogen stimulates cell proliferation in both normal and transformed urothelial cells mainly through activation of the two primary estrogen receptors (ERs), ERalpha and ERbeta. A growing body of evidence suggests that estrogen also initiates nongenomic effects that cannot be explained by activation of primary ERs. In the present study, we observed that urothelial cells express high amounts of GPR30, a G protein-coupled receptor recently identified as a candidate for membrane-associated estrogen binding. Membrane- impermeable bovine serum albumin-conjugated 17beta-estradiol and the specific GPR30 agonist G-1 both inhibited urothelial cell proliferation in a concentration-dependent manner. Transient overexpression of GPR30 inhibited 17beta-estradiol (E2)-induced cell proliferation. Decreased GPR30 expression caused by specific small interfering RNA increased E2-induced cell proliferation. These results indicate that membrane-associated inhibitory effects of E2 on cell proliferation correlate with abundance of GPR30. Although E2 induced a significant increase in caspase-3/7 activity, G-1 did not, suggesting that the GPR30-mediated inhibitory effect on cell proliferation was not caused by apoptosis. Furthermore, we found that G-1 failed to induce c-fos, c-jun, and cyclin D1 expression, and GPR30 overexpression abolished E2-induced c-fos, c-jun, and cyclin D1 expression. However, inactivation of GPR30 by small interfering RNA increased c-fos, c-jun, and cyclin D1 expression. These results suggest that GPR30-mediated inhibition of urothelial cell proliferation is the result of decreased cyclin D1 by down-regulation of activation protein-1 signaling.
Figures






Similar articles
-
G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells.Cancer Res. 2007 Feb 15;67(4):1859-66. doi: 10.1158/0008-5472.CAN-06-2909. Cancer Res. 2007. PMID: 17308128
-
G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage.Am J Pathol. 2007 Apr;170(4):1210-8. doi: 10.2353/ajpath.2007.060883. Am J Pathol. 2007. PMID: 17392161 Free PMC article.
-
The key involvement of estrogen receptor β and G-protein-coupled receptor 30 in the neuroprotective action of daidzein.Neuroscience. 2013 May 15;238:345-60. doi: 10.1016/j.neuroscience.2013.02.005. Epub 2013 Feb 16. Neuroscience. 2013. PMID: 23419549
-
Evidence that the G protein-coupled membrane receptor GPR30 contributes to the cardiovascular actions of estrogen.Gend Med. 2011 Dec;8(6):343-54. doi: 10.1016/j.genm.2011.10.004. Gend Med. 2011. PMID: 22153880 Free PMC article. Review.
-
The ins and outs of GPR30: a transmembrane estrogen receptor.J Steroid Biochem Mol Biol. 2008 Apr;109(3-5):350-3. doi: 10.1016/j.jsbmb.2008.03.006. Epub 2008 Mar 6. J Steroid Biochem Mol Biol. 2008. PMID: 18406603 Free PMC article. Review.
Cited by
-
SMG9 Serves as an Oncogene to Promote the Tumor Progression via EMT and Wnt/β-Catenin Signaling Pathway in Hepatocellular Carcinoma.Front Pharmacol. 2021 Aug 12;12:701454. doi: 10.3389/fphar.2021.701454. eCollection 2021. Front Pharmacol. 2021. PMID: 34456727 Free PMC article.
-
Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30.J Med Chem. 2010 Feb 11;53(3):1004-14. doi: 10.1021/jm9011802. J Med Chem. 2010. PMID: 20041667 Free PMC article.
-
Role of mast cells and protease-activated receptor-2 in cyclooxygenase-2 expression in urothelial cells.Am J Physiol Regul Integr Comp Physiol. 2009 Oct;297(4):R1127-35. doi: 10.1152/ajpregu.00310.2009. Epub 2009 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19675284 Free PMC article.
-
Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30.Curr Top Med Chem. 2009;9(13):1227-36. doi: 10.2174/156802609789753608. Curr Top Med Chem. 2009. PMID: 19807662 Free PMC article. Review.
-
Sex differences in bladder cancer: understanding biological and clinical implications.Biol Sex Differ. 2025 May 13;16(1):31. doi: 10.1186/s13293-025-00715-6. Biol Sex Differ. 2025. PMID: 40361239 Free PMC article. Review.
References
-
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA 1998 The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 19:253–286 - PubMed
-
- Song RX, Santen RJ 2006 Membrane initiated estrogen signaling in breast cancer. Biol Reprod 75:9–16 - PubMed
-
- Mendelsohn ME 2002 Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90:3F–6F - PubMed
-
- Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

