Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats
- PMID: 18467435
- PMCID: PMC2488244
- DOI: 10.1210/en.2008-0439
Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats
Abstract
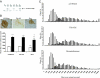
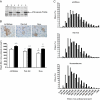
Decrease of muscle IGF-I plays a critical role in muscle atrophy caused by glucocorticoids (GCs) because IGF-I gene electrotransfer prevents muscle atrophy caused by GCs. The goal of the present study was to identify the intracellular mediators responsible for the IGF-I anti-atrophic action in GC-induced muscle atrophy. We first assessed the IGF-I transduction pathway alterations caused by GC administration and their reversibility by local IGF-I overexpression performed by electrotransfer. Muscle atrophy induced by dexamethasone (dexa) administration occurred with a decrease in Akt (-53%; P<0.01) phosphorylation together with a decrease in beta-catenin protein levels (-40%; P<0.001). Prevention of atrophy by IGF-I was associated with restoration of Akt phosphorylation and beta-catenin levels. We then investigated whether muscle overexpression of these intracellular mediators could mimic the IGF-I anti-atrophic effects. Overexpression of a constitutively active form of Akt induced a marked fiber hypertrophy in dexa-treated animals (+175% of cross-sectional area; P<0.001) and prevented dexa-induced atrophy. This hypertrophy was associated with an increase in phosphorylated GSK-3beta (+17%; P<0.05) and in beta-catenin content (+35%; P<0.05). Furthermore, overexpression of a dominant-negative GSK-3beta or a stable form of beta-catenin increased fiber cross-sectional area by, respectively, 23% (P<0.001) and 29% (P<0.001) in dexa-treated rats, preventing completely the atrophic effect of GC. In conclusion, this work indicates that Akt, GSK-3beta, and beta-catenin probably contribute together to the IGF-I anti-atrophic effect in GC-induced muscle atrophy.
Figures



Similar articles
-
Mechanisms of muscle atrophy induced by glucocorticoids.Horm Res. 2009 Nov;72 Suppl 1:36-41. doi: 10.1159/000229762. Epub 2009 Nov 27. Horm Res. 2009. PMID: 19940494 Review.
-
Glycogen synthase kinase-3beta is involved in the process of myocardial hypertrophy stimulated by insulin-like growth factor-1.Circ J. 2004 Mar;68(3):247-53. doi: 10.1253/circj.68.247. Circ J. 2004. PMID: 14993781
-
Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation.Oncogene. 2001 Jan 11;20(2):252-9. doi: 10.1038/sj.onc.1204064. Oncogene. 2001. PMID: 11313952
-
Regulation of signaling pathways downstream of IGF-I/insulin by androgen in skeletal muscle of glucocorticoid-treated rats.J Trauma. 2009 Apr;66(4):1083-90. doi: 10.1097/TA.0b013e31817e7420. J Trauma. 2009. PMID: 19359918 Free PMC article.
-
Glucocorticoid-induced skeletal muscle atrophy.Int J Biochem Cell Biol. 2013 Oct;45(10):2163-72. doi: 10.1016/j.biocel.2013.05.036. Epub 2013 Jun 24. Int J Biochem Cell Biol. 2013. PMID: 23806868 Review.
Cited by
-
Leucine alleviates dexamethasone-induced suppression of muscle protein synthesis via synergy involvement of mTOR and AMPK pathways.Biosci Rep. 2016 Jun 17;36(3):e00346. doi: 10.1042/BSR20160096. Print 2016 Jul. Biosci Rep. 2016. PMID: 27129299 Free PMC article.
-
Serum IGF-1 levels are associated with sarcopenia in elderly men but not in elderly women.Aging Clin Exp Res. 2022 Oct;34(10):2465-2471. doi: 10.1007/s40520-022-02180-2. Epub 2022 Aug 13. Aging Clin Exp Res. 2022. PMID: 35962897
-
Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice.J Clin Invest. 2009 Oct;119(10):3059-69. doi: 10.1172/JCI38770. Epub 2009 Sep 14. J Clin Invest. 2009. PMID: 19759515 Free PMC article.
-
NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma.Carcinogenesis. 2009 Apr;30(4):598-605. doi: 10.1093/carcin/bgp047. Epub 2009 Feb 23. Carcinogenesis. 2009. PMID: 19237607 Free PMC article. Clinical Trial.
-
Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases.Endocr Rev. 2009 Dec;30(7):830-82. doi: 10.1210/er.2009-0013. Epub 2009 Nov 4. Endocr Rev. 2009. PMID: 19890091 Free PMC article. Review.
References
-
- Lecker SH, Solomon V, Mitch WE, Goldberg AL 1999 Muscle protein breakdown and the critical role of the ubiquitin- proteasome pathway in normal and disease states. J Nutr 129(Suppl):227S–237S - PubMed
-
- Hasselgren PO 1999 Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2:201–205 - PubMed
-
- Goldberg AL, Tischler M, DeMartino G, Griffin G 1980 Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed Proc 39:31–36 - PubMed
-
- Lofberg E, Gutierrez A, Wernerman J, Anderstam B, Mitch WE, Price SR, Bergstrom J, Alvestrand A 2002 Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur J Clin Invest 32:345–353 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

