The beta-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus
- PMID: 18467457
- PMCID: PMC2442532
- DOI: 10.1104/pp.107.109512
The beta-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus
Erratum in
- Plant Physiol. 2010 Apr;152(4):2269
Abstract
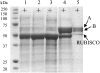
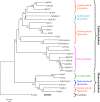
Lotus japonicus accumulates the hydroxynitrile glucosides lotaustralin, linamarin, and rhodiocyanosides A and D. Upon tissue disruption, the hydroxynitrile glucosides are bioactivated by hydrolysis by specific beta-glucosidases. A mixture of two hydroxynitrile glucoside-cleaving beta-glucosidases was isolated from L. japonicus leaves and identified by protein sequencing as LjBGD2 and LjBGD4. The isolated hydroxynitrile glucoside-cleaving beta-glucosidases preferentially hydrolyzed rhodiocyanoside A and lotaustralin, whereas linamarin was only slowly hydrolyzed, in agreement with measurements of their rate of degradation upon tissue disruption in L. japonicus leaves. Comparative homology modeling predicted that LjBGD2 and LjBGD4 had nearly identical overall topologies and substrate-binding pockets. Heterologous expression of LjBGD2 and LjBGD4 in Arabidopsis (Arabidopsis thaliana) enabled analysis of their individual substrate specificity profiles and confirmed that both LjBGD2 and LjBGD4 preferentially hydrolyze the hydroxynitrile glucosides present in L. japonicus. Phylogenetic analyses revealed a third L. japonicus putative hydroxynitrile glucoside-cleaving beta-glucosidase, LjBGD7. Reverse transcription-polymerase chain reaction analysis showed that LjBGD2 and LjBGD4 are expressed in aerial parts of young L. japonicus plants, while LjBGD7 is expressed exclusively in roots. The differential expression pattern of LjBGD2, LjBGD4, and LjBGD7 corresponds to the previously observed expression profile for CYP79D3 and CYP79D4, encoding the two cytochromes P450 that catalyze the first committed step in the biosyntheis of hydroxynitrile glucosides in L. japonicus, with CYP79D3 expression in aerial tissues and CYP79D4 expression in roots.
Figures







References
-
- Ahn YO, Saino H, Mizutani M, Shimizu B, Sakata K (2007) Vicianin hydrolase is a novel cyanogenic β-glycosidase specific to β-vicianoside (6-O-α-L-arabinopyranosyl-β-D-glucopyranoside) in seeds of Vicia angustifolia. Plant Cell Physiol 48 938–947 - PubMed
-
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36 393–405 - PubMed
-
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA (1999) Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J 20 663–671 - PubMed
-
- Bak S, Paquette S, Morant M, Morant AV, Saito S, Bjarnholt N, Zagrobelny M, Jørgensen K, Osmani S, Hamann T, et al (2006) Cyanogenic glucosides: a case study for evolution and application of cytochromes P450. Phytochem Rev 5 309–329
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

