Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons
- PMID: 18467592
- PMCID: PMC3429343
- DOI: 10.1126/science.1156093
Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons
Abstract
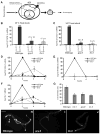
Temperature pervasively affects all cellular processes. In response to a rapid increase in temperature, all cells undergo a heat shock response, an ancient and highly conserved program of stress-inducible gene expression, to reestablish cellular homeostasis. In isolated cells, the heat shock response is initiated by the presence of misfolded proteins and therefore thought to be cell-autonomous. In contrast, we show that within the metazoan Caenorhabditis elegans, the heat shock response of somatic cells is not cell-autonomous but rather depends on the thermosensory neuron, AFD, which senses ambient temperature and regulates temperature-dependent behavior. We propose a model whereby this loss of cell autonomy serves to integrate behavioral, metabolic, and stress-related responses to establish an organismal response to environmental change.
Figures
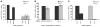
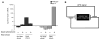
Similar articles
-
Heat shock factor 1 prevents the reduction in thrashing due to heat shock in Caenorhabditis elegans.Biochem Biophys Res Commun. 2015 Jul 3;462(3):190-4. doi: 10.1016/j.bbrc.2015.04.086. Epub 2015 Apr 29. Biochem Biophys Res Commun. 2015. PMID: 25935486
-
Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis.EMBO Rep. 2011 Jul 8;12(8):855-62. doi: 10.1038/embor.2011.120. EMBO Rep. 2011. PMID: 21738224 Free PMC article.
-
A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans.J Neurosci. 2013 Apr 3;33(14):6102-11. doi: 10.1523/JNEUROSCI.4023-12.2013. J Neurosci. 2013. PMID: 23554491 Free PMC article.
-
The small heat shock proteins of the nematode Caenorhabditis elegans: structure, regulation and biology.Prog Mol Subcell Biol. 2002;28:61-78. doi: 10.1007/978-3-642-56348-5_4. Prog Mol Subcell Biol. 2002. PMID: 11908066 Review. No abstract available.
-
Regulatory systems that mediate the effects of temperature on the lifespan of Caenorhabditis elegans.J Neurogenet. 2020 Sep-Dec;34(3-4):518-526. doi: 10.1080/01677063.2020.1781849. Epub 2020 Jul 7. J Neurogenet. 2020. PMID: 32633588 Review.
Cited by
-
Disease tolerance as a defense strategy.Science. 2012 Feb 24;335(6071):936-41. doi: 10.1126/science.1214935. Science. 2012. PMID: 22363001 Free PMC article. Review.
-
Diversity in the origins of proteostasis networks--a driver for protein function in evolution.Nat Rev Mol Cell Biol. 2013 Apr;14(4):237-48. doi: 10.1038/nrm3542. Epub 2013 Mar 6. Nat Rev Mol Cell Biol. 2013. PMID: 23463216 Free PMC article. Review.
-
Heterotypic Signals from Neural HSF-1 Separate Thermotolerance from Longevity.Cell Rep. 2015 Aug 18;12(7):1196-1204. doi: 10.1016/j.celrep.2015.07.026. Epub 2015 Aug 6. Cell Rep. 2015. PMID: 26257177 Free PMC article.
-
Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila.Genes Dev. 2010 Nov 1;24(21):2365-82. doi: 10.1101/gad.1953710. Genes Dev. 2010. PMID: 21041406 Free PMC article. Review.
-
The good and the bad of being connected: the integrons of aging.Curr Opin Cell Biol. 2014 Feb;26:107-12. doi: 10.1016/j.ceb.2013.12.003. Epub 2013 Dec 30. Curr Opin Cell Biol. 2014. PMID: 24529252 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

