Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice
- PMID: 18467653
- PMCID: PMC2493075
- DOI: 10.1189/jlb.1207816
Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice
Abstract
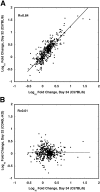
Pneumocystis is a pathogen of immunocompromised hosts but can also infect healthy hosts, in whom infection is rapidly controlled and cleared. Microarray methods were used to examine differential gene expression in the lungs of C57BL/6 and CD40 ligand knockout (CD40L-KO) mice over time following exposure to Pneumocystis murina. Immunocompetent C57BL/6 mice, which control and clear infection efficiently, showed a robust response to infection characterized by the up-regulation of 349 primarily immune response-associated genes. Temporal changes in the expression of these genes identified an early (Week 2), primarily innate response, which waned before the infection was controlled; this was followed by primarily adaptive immune responses that peaked at Week 5, which coincided with clearance of the infection. In conjunction with the latter, there was an increased expression of B cell-associated (Ig) genes at Week 6 that persisted through 11 weeks. In contrast, CD40L-KO mice, which are highly susceptible to developing severe Pneumocystis pneumonia, showed essentially no up-regulation of immune response-associated genes at Days 35-75. Immunohistochemical staining supported these observations by demonstrating an increase in CD4+, CD68+, and CD19+ cells in C57BL/6 but not CD40L-KO mice. Thus, the healthy host demonstrates a robust, biphasic response to infection by Pneumocystis; CD40L is an essential upstream regulator of the adaptive immune responses that efficiently control infection and prevent development of progressive pneumonia.
Figures




References
-
- Kovacs J A, Gill V J, Meshnick S, Masur H. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA. 2001;286:2450–2460. - PubMed
-
- Bishop L R, Kovacs J A. Quantitation of anti-Pneumocystis jiroveci antibodies in healthy persons and immunocompromised patients. J Infect Dis. 2003;187:1844–1848. - PubMed
-
- Meuwissen J H, Tauber I, Leeuwenberg A D, Beckers P J, Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977;136:43–49. - PubMed
-
- Steele C, Shellito J E, Kolls J K. Immunity against the opportunistic fungal pathogen Pneumocystis. Med Mycol. 2005;43:1–19. - PubMed
-
- Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

