Macrophages define the invasive microenvironment in breast cancer
- PMID: 18467655
- PMCID: PMC2516896
- DOI: 10.1189/jlb.1107762
Macrophages define the invasive microenvironment in breast cancer
Abstract
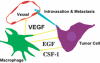
In many human cancers, the abundance of macrophages in the tumor microenvironment is correlated with poor prognosis. Experimental evidence for the causal relationship between macrophages and poor prognosis came from mouse models of breast cancer in which genetic ablation of macrophages resulted in attenuation of tumor progression and metastasis, and premature recruitment to hyperplastic lesions accelerated these processes. Malignancy is defined by the invasion of tumor cells into the stroma, a process that allows escape of these cells into the circulation and dissemination to distant sites. In this review, I argue that macrophages are recruited to the invasive front by expression of tumor-derived chemotactic factors and in response to the disruption of the basement membrane. At this invasive site, macrophages enhance tumor cell migration and invasion through their secretion of chemotactic and chemokinetic factors including epidermal growth factor (EGF). They promote angiogenesis by the synthesis of angiogenic factors including vascular endothelial growth factor (VEGF), and they remodel the extracellular matrix and in particular, regulate collagen fibrillogenesis. A combination of these factors provides a triple-whammy, as the more mobile and invasive tumor cells track along collagen fibers that are also anchored to blood vessels, which are fabricated at sites of invasion and through which macrophages potentiate tumor cell intravasation. All of these activities suggest that macrophage functions are significant targets for the generation of novel therapeutics that should improve the current cytotoxic armamentarium.
Figures
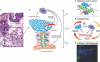
References
-
- Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Martins-Green M, Boudreau N, Bissell M J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Iyengar P, Combs T P, Shah S J, Gouon-Evans V, Pollard J W, Albanese C, Flanagan L, Tenniswood M P, Guha C, Lisanti M P, Pestell R G, Scherer P E. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

