Hepatocyte nuclear factor-1 as marker of epithelial phenotype reveals marrow-derived hepatocytes, but not duct cells, after liver injury in mice
- PMID: 18467658
- PMCID: PMC2846397
- DOI: 10.1634/stemcells.2008-0148
Hepatocyte nuclear factor-1 as marker of epithelial phenotype reveals marrow-derived hepatocytes, but not duct cells, after liver injury in mice
Abstract
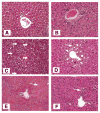
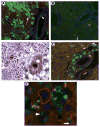
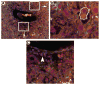
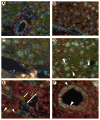
The potential bone marrow origin of hepatocytes, cholangiocytes, and ductal progenitor cells in the liver was examined in female mice after transplantation of bone marrow cells from male green fluorescent protein (GFP) transgenic donors. Following stable hematopoietic engraftment, the livers of the recipients were injured with carbon tetrachloride (CCl(4), with or without local irradiation of the liver) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC, with or without local irradiation of the liver). The presence of numerous marrow-derived, GFP-positive inflammatory cells had the potential to lead to erroneous interpretation of marrow-derived hepatocytes, cholangiocytes, and ductal progenitor cells. Identification of marrow-derived ductal progenitor or cholangiocyte phenotype using colocalization of GFP or Y chromosome with pancytokeratin staining also failed to distinguish epithelial cells from closely apposed inflammatory cells. To address this inadequacy, we developed a rigorous new immunofluorescence protocol to identify marrow-derived epithelial cells in the liver using Y chromosome (donor marker) and hepatocyte nuclear factor-1 (HNF1, a nuclear marker of liver epithelial, nonhematopoietic phenotype). Using the Y/HNF1 method, rare (approximately one in 20,000) hepatocytes in female mice transplanted with male bone marrow contained a donor-derived Y chromosome. On the other hand, no Y chromosomes were found in cholangiocytes or ductal progenitor cells in mice with liver injury due to DDC or CCl(4). The use of a nuclear marker of mature hepatocytes or cholangiocytes, such as HNF1, improves discrimination of marrow-derived epithelial cells in tissue sections.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


Similar articles
-
Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver.Am J Pathol. 2005 Aug;167(2):555-64. doi: 10.1016/S0002-9440(10)62997-5. Am J Pathol. 2005. PMID: 16049339 Free PMC article.
-
Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes.Cell Tissue Res. 2006 Feb;323(2):221-31. doi: 10.1007/s00441-005-0077-0. Epub 2005 Oct 14. Cell Tissue Res. 2006. PMID: 16228231
-
Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver.Proc Natl Acad Sci U S A. 2003 Sep 30;100 Suppl 1(Suppl 1):11850-3. doi: 10.1073/pnas.1834198100. Epub 2003 Aug 14. Proc Natl Acad Sci U S A. 2003. PMID: 12920184 Free PMC article.
-
Detection of bone marrow-derived lung epithelial cells.Exp Hematol. 2010 Jul;38(7):564-73. doi: 10.1016/j.exphem.2010.04.011. Epub 2010 May 4. Exp Hematol. 2010. PMID: 20447442 Free PMC article. Review.
-
Lineage plasticity and reprogramming of epithelial cells during tissue injury and regeneration-lessons from the lineage plasticity of hepatocytes and cholangiocytes induced by liver injury.Regen Ther. 2025 Apr 22;29:447-454. doi: 10.1016/j.reth.2025.04.008. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40487923 Free PMC article. Review.
Cited by
-
Survival of irradiated recipient mice after transplantation of bone marrow from young, old and "early aging" mice.Aging (Albany NY). 2015 Dec;7(12):1212-23. doi: 10.18632/aging.100867. Aging (Albany NY). 2015. PMID: 26796640 Free PMC article.
-
Treatment of newborn G6pc(-/-) mice with bone marrow-derived myelomonocytes induces liver repair.J Hepatol. 2011 Dec;55(6):1263-71. doi: 10.1016/j.jhep.2011.02.033. Epub 2011 Apr 13. J Hepatol. 2011. PMID: 21703205 Free PMC article.
-
The impact of hepatocyte nuclear factor-1α on liver malignancies and cell stemness with metabolic consequences.Stem Cell Res Ther. 2019 Nov 4;10(1):315. doi: 10.1186/s13287-019-1438-z. Stem Cell Res Ther. 2019. PMID: 31685031 Free PMC article. Review.
-
Apolipoprotein M could inhibit growth and metastasis of SMMC7721 cells via vitamin D receptor signaling.Cancer Manag Res. 2019 Apr 30;11:3691-3701. doi: 10.2147/CMAR.S202799. eCollection 2019. Cancer Manag Res. 2019. PMID: 31190977 Free PMC article.
-
Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model.Hepatology. 2009 Jun;49(6):1992-2000. doi: 10.1002/hep.22862. Hepatology. 2009. PMID: 19437487 Free PMC article.
References
-
- Dexter TM, Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. - PubMed
-
- Orkin S. Hematopoietic stem cells: Molecular diversification and developmental interrelationships. In: Marshak D, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 289–306.
-
- Bonnet DA. Haematopoietic stem cells. J Pathol. 2002;197:430–440. - PubMed
-
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. - PubMed
-
- Eisenberg LM, Eisenberg CA. Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Res C Embryo Today Rev. 2003;69:209–218. - PubMed

