Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis
- PMID: 18467701
- PMCID: PMC2408419
- DOI: 10.2353/ajpath.2008.071067
Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis
Abstract
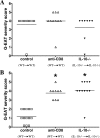
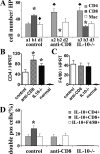
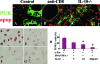
Granulomatous experimental autoimmune thyroiditis (G-EAT) is induced by mouse thyroglobulin-sensitized splenocytes activated in vitro with mouse thyroglobulin and interleukin (IL)-12. Thyroid lesions reach maximal severity 20 days after cell transfer, and inflammation either resolves or progresses to fibrosis by day 60 depending on the extent of thyroid damage at day 20. Depletion of CD8(+) T cells inhibits G-EAT resolution. Our previous studies indicated that IL-10 was generally higher in G-EAT thyroids that resolved. Using both wild-type and IL-10(-/-) CBA/J mice, this study was undertaken to determine whether G-EAT resolution would be inhibited in the absence of IL-10. The results showed that either depletion of CD8(+) T cells or IL-10 deficiency increased fibrosis and inhibited resolution of inflammation. We also found a correlation between higher expression levels of proinflammatory cytokines and preferential expression levels of proapoptotic molecules, such as FasL and TRAIL, and antiapoptotic molecules, such as FLIP and Bcl-xL, in inflammatory cells from thyroids of both CD8-depleted and IL-10-deficient mice. Furthermore, many of the CD8(+) T cells were also IL-10(+). These results suggest that IL-10 plays an important role in G-EAT resolution and might promote resolution, at least in part, through its production in CD8(+) T cells. Further understanding of the mechanisms that promote the resolution of inflammation will facilitate the development of novel strategies for treating autoimmune diseases.
Figures




Similar articles
-
Eosinophils infiltrate thyroids, but have no apparent role in induction or resolution of experimental autoimmune thyroiditis in interferon-gamma(-/-) mice.Immunology. 2010 Mar;129(3):329-37. doi: 10.1111/j.1365-2567.2009.03187.x. Epub 2009 Oct 21. Immunology. 2010. PMID: 19845793 Free PMC article.
-
Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice.Endocrinology. 2007 Dec;148(12):5734-45. doi: 10.1210/en.2007-0939. Epub 2007 Sep 6. Endocrinology. 2007. PMID: 17823262
-
Apoptosis of thyrocytes and effector cells during induction and resolution of granulomatous experimental autoimmune thyroiditis.Int Immunol. 2000 Dec;12(12):1629-39. doi: 10.1093/intimm/12.12.1629. Int Immunol. 2000. PMID: 11099302
-
Expression and regulation of Fas and Fas ligand on thyrocytes and infiltrating cells during induction and resolution of granulomatous experimental autoimmune thyroiditis.J Immunol. 2001 Dec 1;167(11):6678-86. doi: 10.4049/jimmunol.167.11.6678. J Immunol. 2001. PMID: 11714840
-
Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis.Int Rev Immunol. 2000;19(6):535-55. doi: 10.3109/08830180009088511. Int Rev Immunol. 2000. PMID: 11129114 Review.
Cited by
-
Blueberry as a Potential Radiosensitizer for Treating Cervical Cancer.Pathol Oncol Res. 2019 Jan;25(1):81-88. doi: 10.1007/s12253-017-0319-y. Epub 2017 Sep 30. Pathol Oncol Res. 2019. PMID: 28963664
-
A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis.J Pathol. 2008 Dec;216(4):505-13. doi: 10.1002/path.2428. J Pathol. 2008. PMID: 18810759 Free PMC article.
-
Eosinophils infiltrate thyroids, but have no apparent role in induction or resolution of experimental autoimmune thyroiditis in interferon-gamma(-/-) mice.Immunology. 2010 Mar;129(3):329-37. doi: 10.1111/j.1365-2567.2009.03187.x. Epub 2009 Oct 21. Immunology. 2010. PMID: 19845793 Free PMC article.
-
Resolution of inflammation: a new therapeutic frontier.Nat Rev Drug Discov. 2016 Aug;15(8):551-67. doi: 10.1038/nrd.2016.39. Epub 2016 Mar 29. Nat Rev Drug Discov. 2016. PMID: 27020098 Review.
-
Effect of transgenic overexpression of FLIP on lymphocytes on development and resolution of experimental autoimmune thyroiditis.Am J Pathol. 2011 Sep;179(3):1211-20. doi: 10.1016/j.ajpath.2011.05.054. Epub 2011 Jul 16. Am J Pathol. 2011. PMID: 21763264 Free PMC article.
References
-
- Conaway DH, Giraldo AA, David CS, Kong YC. In situ analysis of T cell subset composition in experimental autoimmune thyroiditis after adoptive transfer of activated spleen cells. Cell Immunol. 1990;125:247–253. - PubMed
-
- Lira SA, Martin AP, Marinkovic T, Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol. 2005;25:251–262. - PubMed
-
- Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985;93:132–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

