By homing to the kidney, activated macrophages potently exacerbate renal injury
- PMID: 18467704
- PMCID: PMC2408410
- DOI: 10.2353/ajpath.2008.070825
By homing to the kidney, activated macrophages potently exacerbate renal injury
Erratum in
- Am J Pathol. 2008 Dec;173(6):1929. Cai, Qi [corrected to Cao, Qi]
Abstract
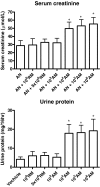
Macrophages are important mediators of injury in most types of human kidney diseases; however, the pathogenic importance of both macrophage number and activation status is unknown. To examine this question, severe-combined immunodeficient mice with adriamycin nephrosis, an experimental model of human focal segmental glomerulosclerosis, were treated intravenously with either resting (1 x 10(6) to 5 x 10(6)) or activated (1 x 10(3) to 1 x 10(6)) macrophages on day 6 postadriamycin administration, and the effects on kidney injury were examined. On day 28, renal injury was worse in the group that received activated macrophages at doses as low as 1 x 10(4) macrophages per mouse compared with control adriamycin nephrotic mice. However, treatment with resting macrophages at doses as high as 5 x 10(6) macrophages per mouse had no significant effect on either renal histology or function. The transferred activated macrophages homed to inflamed kidneys during the middle-to-late stages of the disease, but such homing was not observed for resting macrophages. This study of in vivo cell adoptive transfer supports the importance of macrophage activation status over macrophage number in causing renal injury. These data suggest that therapeutic strategies for treating progressive kidney diseases should target activated macrophages.
Figures




Similar articles
-
Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease.Kidney Int. 2007 Aug;72(3):290-9. doi: 10.1038/sj.ki.5002275. Epub 2007 Apr 18. Kidney Int. 2007. PMID: 17440493
-
The CD40-CD154 co-stimulation pathway mediates innate immune injury in adriamycin nephrosis.Nephrol Dial Transplant. 2010 Mar;25(3):717-30. doi: 10.1093/ndt/gfp569. Epub 2009 Nov 4. Nephrol Dial Transplant. 2010. PMID: 19889873
-
Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease.Kidney Int. 2013 Oct;84(4):745-55. doi: 10.1038/ki.2013.135. Epub 2013 May 1. Kidney Int. 2013. PMID: 23636175
-
IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease.J Am Soc Nephrol. 2011 Jul;22(7):1229-39. doi: 10.1681/ASN.2010070693. Epub 2011 Jun 30. J Am Soc Nephrol. 2011. PMID: 21719780 Free PMC article.
-
Adriamycin nephropathy: a model of focal segmental glomerulosclerosis.Nephrology (Carlton). 2011 Jan;16(1):30-8. doi: 10.1111/j.1440-1797.2010.01383.x. Nephrology (Carlton). 2011. PMID: 21175974 Review.
Cited by
-
The renal mononuclear phagocytic system.J Am Soc Nephrol. 2012 Feb;23(2):194-203. doi: 10.1681/ASN.2011070680. Epub 2011 Dec 1. J Am Soc Nephrol. 2012. PMID: 22135312 Free PMC article. Review.
-
Macrophage heterogeneity, phenotypes, and roles in renal fibrosis.Kidney Int Suppl (2011). 2014 Nov;4(1):16-19. doi: 10.1038/kisup.2014.4. Kidney Int Suppl (2011). 2014. PMID: 26312145 Free PMC article. Review.
-
Infiltrating macrophages and interferon gamma rather than renal genotype dictate heightened crescentic glomerulonephritis.Front Immunol. 2024 Dec 19;15:1484525. doi: 10.3389/fimmu.2024.1484525. eCollection 2024. Front Immunol. 2024. PMID: 39749339 Free PMC article.
-
Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments.Mediators Inflamm. 2012;2012:951390. doi: 10.1155/2012/951390. Epub 2012 Dec 3. Mediators Inflamm. 2012. PMID: 23251037 Free PMC article. Review.
-
Bee Venom Mitigates Cisplatin-Induced Nephrotoxicity by Regulating CD4(+)CD25(+)Foxp3(+) Regulatory T Cells in Mice.Evid Based Complement Alternat Med. 2013;2013:879845. doi: 10.1155/2013/879845. Epub 2013 Feb 14. Evid Based Complement Alternat Med. 2013. PMID: 23476708 Free PMC article.
References
-
- Ferrario F, Castiglione A, Colasanti G, Barbiano di Belgioioso G, Bertoli S, D'Amico G. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28:513–519. - PubMed
-
- Schreiner GF. Macrophages and cellular immunity in experimental nephrosis and glomerulonephritis. Contrib Nephrol. 1985;45:115–122. - PubMed
-
- Atkins RC. Macrophages in renal injury. Am J Kidney Dis. 1998;31:xlv–xlvii. - PubMed
-
- Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol. 2003;170:3254–3262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

