Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma
- PMID: 18467720
- PMCID: PMC3724516
- DOI: 10.1200/JCO.2007.13.3165
Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma
Abstract
Purpose: High-dose interleukin-2 (IL-2) induces responses in 15% to 20% of patients with advanced melanoma; 5% to 8% are durable complete responses (CRs). The HLA-A2-restricted, modified gp100 peptide (210M) induces T-cell immunity in vivo and has little antitumor activity but, combined with high-dose IL-2, reportedly has a 42% (13 of 31 patients) response rate (RR). We evaluated 210M with one of three different IL-2 schedules to determine whether a basis exists for a phase III trial.
Patients and methods: In three separate phase II trials, patients with melanoma received 210M subcutaneously during weeks 1, 4, 7, and 10 and standard high-dose IL-2 during weeks 1 and 3 (trial 1), weeks 7 and 9 (trial 2), or weeks 1, 4, 7, and 10 (trial 3). Immune assays were performed on peripheral-blood mononuclear cells collected before and after treatment.
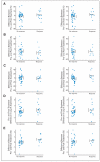
Results: From 1998 to 2003, 131 patients with HLA-A2-positive were enrolled. With 60-month median follow-up time, the overall RR for 121 assessable patients was 16.5% (95% CI, 10% to 26%); the RRs were 23.8% in trial 1 (42 patients), 12.5% in trial 2 (40 patients), and 12.8% in trial 3 (39 patients). There were 11 CRs (9%) and nine partial responses (7%), with 11 patients (9%) progression free at >or= 30 months. Immune studies including assays of CD3-zeta expression and numbers of CD4(+)/CD25(+)/FoxP3(+) regulatory T cells, CD15(+)/CD11b(+)/CD14(-) immature myeloid-derived cells, and CD8(+)gp100 tetramer-positive cells in the blood did not correlate with clinical benefit.
Conclusion: The results again demonstrate efficacy of high-dose IL-2 in advanced melanoma but did not demonstrate the promising clinical activity reported with vaccine and high-dose IL-2 in any of three phase II trials.
Figures

Comment in
-
Combining a peptide vaccine with high-dose interleukin-2.J Clin Oncol. 2008 May 10;26(14):2250-1. doi: 10.1200/JCO.2007.15.7826. J Clin Oncol. 2008. PMID: 18467715 No abstract available.
-
"Groovy" vaccine for melanoma--but which groove?J Clin Oncol. 2008 Dec 20;26(36):6009-10; author reply 6010-1. doi: 10.1200/JCO.2008.19.4886. Epub 2008 Nov 17. J Clin Oncol. 2008. PMID: 19018078 No abstract available.
References
-
- Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: What have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. - PubMed
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
-
- Sosman JA, Weiss GR, Margolin KA, et al. Phase IB clinical trial of anti-CD3 followed by high-dose bolus interleukin-2 in patients with metastatic melanoma and advanced renal cell carcinoma: Clinical and immunologic effects. J Clin Oncol. 1993;11:1496–1505. - PubMed
-
- Whitehead RP, Friedman KD, Clark DA, et al. Phase I trial of simultaneous administration of interleukin 2 and interleukin 4 subcutaneously. Clin Cancer Res. 1995;1:1145–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

