Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups
- PMID: 18467730
- PMCID: PMC4558625
- DOI: 10.1200/JCO.2007.14.7207
Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups
Abstract
Purpose: To identify risk factors associated with outcome in children with metastatic rhabdomyosarcoma in a large cohort of patients
Patients and methods: Pooled data were obtained from 788 patients treated in nine studies performed by European and American cooperative groups. Clinical factors, including age, histology, site of primary, and site(s) and number of sites of metastatic disease, were correlated with event-free survival (EFS) and overall survival (OS).
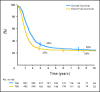
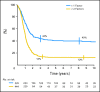
Results: Seven hundred eighty-eight patients were eligible for analysis. The 3-year OS and EFS were 34% (SE, 1.7) and 27% (SE, 1.6), respectively. By univariate analysis, 3-year EFS was significantly and adversely influenced by age, alveolar histology, location of primary tumor in unfavorable site (defined as extremity and "other" sites), presence of three or more sites of metastatic disease, and the presence of bone or bone marrow involvement. By multivariate analysis, EFS was strongly correlated to all factors except histology. Relative risks were 1.6 for age younger than 1 year or at least 10 years, 1.4 for unfavorable site of primary tumor, 1.4 for bone or bone marrow involvement, 1.4 for three or more metastatic sites. EFS was 50% for patients without any of these four adverse factors and was respectively 42%, 18%, 12%, and 5% in patients with one, two, three, or four factors (P < .0001).
Conclusion: This analysis identified subsets of patients with metastatic rhabdomyosarcoma with different outcomes to current therapy and offers a strategy to define patient candidates for experimental approaches to treatment.
Figures
References
-
- Crist W, Gehan EA, Ragab AH, et al: The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 13:610-630, 1995 - PubMed
-
- Koscielniak E, Harms D, Henze G, et al: Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17:3706-3719, 1999 - PubMed
-
- Stevens MC, Rey A, Bouvet N, et al: Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology–SIOP malignant mesenchymal tumor 89. J Clin Oncol 23:2618-2628, 2005 - PubMed
-
- Breitfeld PP, Lyden E, Raney RB, et al: Ifosfamide and etoposide are superior to vincristine and melphalan for pediatric metastatic rhabdomyosarcoma when administered with irradiation and combination chemotherapy: A report from the Intergroup Rhabdomyosarcoma Study Group. J Pediatr Hematol Oncol 23:225-233, 2001 - PubMed
-
- Breneman JC, Lyden E, Pappo AS, et al: Prognostic factors and clinical outcomes in children and adolescents with metastatic Rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 21:78-84, 2003 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources