Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction
- PMID: 18468463
- PMCID: PMC2394854
- DOI: 10.1016/j.immuni.2008.03.016
Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction
Abstract
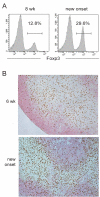
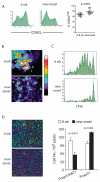
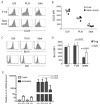
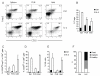
The dynamics of CD4(+) effector T cells (Teff cells) and CD4(+)Foxp3(+) regulatory T cells (Treg cells) during diabetes progression in nonobese diabetic mice was investigated to determine whether an imbalance of Treg cells and Teff cells contributes to the development of type 1 diabetes. Our results demonstrated a progressive decrease in the Treg cell:Teff cell ratio in inflamed islets but not in pancreatic lymph nodes. Intra-islet Treg cells expressed reduced amounts of CD25 and Bcl-2, suggesting that their decline was due to increased apoptosis. Additionally, administration of low-dose interleukin-2 (IL-2) promoted Treg cell survival and protected mice from developing diabetes. Together, these results suggest intra-islet Treg cell dysfunction secondary to defective IL-2 production is a root cause of the progressive breakdown of self-tolerance and the development of diabetes in nonobese diabetic mice.
Figures


Comment in
-
Regulating Treg cells at sites of inflammation.Immunity. 2008 Oct 17;29(4):511; author reply 512. doi: 10.1016/j.immuni.2008.09.012. Immunity. 2008. PMID: 18957259 No abstract available.
References
-
- Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev. 2006;212:330–343. - PubMed
-
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. - PubMed
-
- Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–2702. - PubMed
-
- Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212:287–300. - PubMed
-
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

