Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes
- PMID: 18468992
- PMCID: PMC2734298
- DOI: 10.1093/toxsci/kfn093
Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes
Abstract
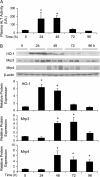
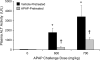
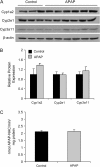
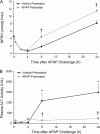
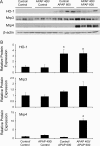
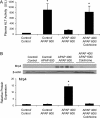
Treatment with hepatotoxicants such as acetaminophen (APAP) causes resistance to a second, higher dose of the same toxicant (autoprotection). APAP induces hepatic mRNA and protein levels of the multidrug resistance-associated proteins (Mrp) transporters in mice and humans. Basolateral efflux transporters Mrp3 and Mrp4 are the most significantly induced. We hypothesized that upregulation of Mrp3 and Mrp4 is one mechanism by which hepatocytes become resistant to a subsequent higher dose of APAP by limiting accumulation of xeno-, endobiotics, and byproducts of hepatocellular injury. The purpose of this study was to evaluate Mrp3 and Mrp4 expression in proliferating hepatocytes in a mouse model of APAP autoprotection. Plasma and livers were collected from male C57BL/6J mice treated with APAP 400 mg/kg for determination of hepatotoxicity and protein expression. Maximal Mrp3 and Mrp4 induction occurred 48 h after APAP. Mrp4 upregulation occurred selectively in proliferating hepatocytes. Additional groups of APAP-pretreated mice were challenged 48 h later with a second, higher dose of APAP. APAP-pretreated mice had reduced hepatotoxicity after APAP challenge compared to those pretreated with vehicle. A more rapid recovery of glutathione (GSH) in APAP-pretreated mice corresponded with increases in GSH synthetic enzymes. Interestingly, mice pretreated and challenged with APAP had dramatic increases in Mrp4 expression as well as enhanced hepatocyte proliferation. Inhibition of hepatocyte replication with colchicine not only restored sensitivity of APAP-pretreated mice to injury, but also blocked Mrp4 induction. Mrp4 overexpression may be one phenotypic property of proliferating hepatocytes that protects against subsequent hepatotoxicant exposure by mechanisms that are presently unknown.
Figures




References
-
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol. Sci. 2006;89:370–379. - PubMed
-
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol. Sci. 2005;83:44–52. - PubMed
-
- Bai J, Lai L, Yeo HC, Goh BC, Tan TM. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int. J. Biochem. Cell. Biol. 2004;36:247–257. - PubMed
-
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J. Pharmacol. Exp. Ther. 2003;307:67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

