Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome
- PMID: 18468998
- PMCID: PMC2441552
- DOI: 10.1074/jbc.M801860200
Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome
Abstract
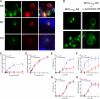
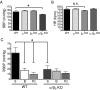
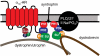
Hypertension is a cardiovascular disease associated with increased plasma catecholamines, overactivation of the sympathetic nervous system, and increased vascular tone and total peripheral resistance. A key regulator of sympathetic nervous system function is the alpha(1D)-adrenergic receptor (AR), which belongs to the adrenergic family of G-protein-coupled receptors (GPCRs). Endogenous catecholamines norepinephrine and epinephrine activate alpha(1D)-ARs on vascular smooth muscle to stimulate vasoconstriction, which increases total peripheral resistance and mean arterial pressure. Indeed, alpha(1D)-AR KO mice display a hypotensive phenotype and are resistant to salt-induced hypertension. Unfortunately, little information exists about how this important GPCR functions because of an inability to obtain functional expression in vitro. Here, we identified the dystrophin proteins, syntrophin, dystrobrevin, and utrophin as essential GPCR-interacting proteins for alpha(1D)-ARs. We found that dystrophins complex with alpha(1D)-AR both in vitro and in vivo to ensure proper functional expression. More importantly, we demonstrate that knock-out of multiple syntrophin isoforms results in the complete loss of alpha(1D)-AR function in mouse aortic smooth muscle cells and abrogation of alpha(1D)-AR-mediated increases in blood pressure. Our findings demonstrate that syntrophin and utrophin associate with alpha(1D)-ARs to create a functional signalosome, which is essential for alpha(1D)-AR regulation of vascular tone and blood pressure.
Figures

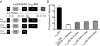



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

