Breaking up is hard to do - membrane traffic in cytokinesis
- PMID: 18469013
- PMCID: PMC4365974
- DOI: 10.1242/jcs.018770
Breaking up is hard to do - membrane traffic in cytokinesis
Abstract
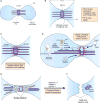
Throughout normal development, and in aberrant conditions such as cancer, cells divide by a process called cytokinesis. Most textbooks suggest that animal cells execute cytokinesis using an actomyosin-containing contractile ring, whereas plant cells generate a new cell wall by the assembly of a novel membrane compartment using vesicle-trafficking machinery in an apparently distinct manner. Recent studies have shown that cytokinesis in animal and plant cells may not be as distinct as these models imply - both have an absolute requirement for vesicle traffic. Moreover, some of the key molecular components of cytokinesis have been identified, many of which are proteins that function to control membrane traffic. Here, we review recent advances in this area.
Figures
References
-
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. - PubMed
-
- Baluska F, Menzel D, Barlow PW. Cytokinesis in plant and animal cells: endosomes ‘shut the door’. Dev Biol. 2006;294:1–10. - PubMed
-
- Barr FA, Greneberg U. Cytokinesis: Placing and making the final cut. Cell. 2007;131:847–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

