The RanGTP gradient - a GPS for the mitotic spindle
- PMID: 18469014
- PMCID: PMC7185306
- DOI: 10.1242/jcs.005959
The RanGTP gradient - a GPS for the mitotic spindle
Abstract
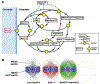
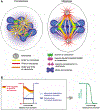
The GTPase Ran has a key role in nuclear import and export, mitotic spindle assembly and nuclear envelope formation. The cycling of Ran between its GTP- and GDP-bound forms is catalyzed by the chromatin-bound guanine nucleotide exchange factor RCC1 and the cytoplasmic Ran GTPase-activating protein RanGAP. The result is an intracellular concentration gradient of RanGTP that equips eukaryotic cells with a ;genome-positioning system' (GPS). The binding of RanGTP to nuclear transport receptors (NTRs) of the importin beta superfamily mediates the effects of the gradient and generates further downstream gradients, which have been elucidated by fluorescence resonance energy transfer (FRET) imaging and computational modeling. The Ran-dependent GPS spatially directs many functions required for genome segregation by the mitotic spindle during mitosis. Through exportin 1, RanGTP recruits essential centrosome and kinetochore components, whereas the RanGTP-induced release of spindle assembly factors (SAFs) from importins activates SAFs to nucleate, bind and organize nascent spindle microtubules. Although a considerable fraction of cytoplasmic SAFs is active and RanGTP induces only partial further activation near chromatin, bipolar spindle assembly is robustly induced by cooperativity and positive-feedback mechanisms within the network of Ran-activated SAFs. The RanGTP gradient is conserved, although its roles vary among different cell types and species, and much remains to be learned regarding its functions.
Figures

References
-
- Albee AJ, Tao W and Wiese C (2006). Phosphorylation of maskin by Aurora-A is regulated by RanGTP and importin beta. J. Biol. Chem 281, 38293–38301. - PubMed
-
- Altieri DC (2006). The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol 18, 609–615. - PubMed
-
- Arnaoutov A and Dasso M (2005). Ran-GTP regulates kinetochore attachment in somatic cells. Cell Cycle 4, 1161–1165. - PubMed
-
- Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J and Dasso M (2005). Crml is a mitotic effector of Ran-GTP in somatic cells. Nat. Cell Biol 7, 626–632. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

