Central control of thermogenesis in mammals
- PMID: 18469069
- PMCID: PMC2496891
- DOI: 10.1113/expphysiol.2007.041848
Central control of thermogenesis in mammals
Abstract
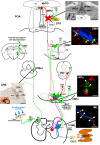
Thermogenesis, the production of heat energy, is an essential component of the homeostatic repertoire to maintain body temperature in mammals and birds during the challenge of low environmental temperature and plays a key role in elevating body temperature during the febrile response to infection. The primary sources of neurally regulated metabolic heat production are mitochondrial oxidation in brown adipose tissue, increases in heart rate and shivering in skeletal muscle. Thermogenesis is regulated in each of these tissues by parallel networks in the central nervous system, which respond to feedforward afferent signals from cutaneous and core body thermoreceptors and to feedback signals from brain thermosensitive neurons to activate the appropriate sympathetic and somatic efferents. This review summarizes the research leading to a model of the feedforward reflex pathway through which environmental cold stimulates thermogenesis and discusses the influence on this thermoregulatory network of the pyrogenic mediator, prostaglandin E(2), to increase body temperature. The cold thermal afferent circuit from cutaneous thermal receptors ascends via second-order thermosensory neurons in the dorsal horn of the spinal cord to activate neurons in the lateral parabrachial nucleus, which drive GABAergic interneurons in the preoptic area to inhibit warm-sensitive, inhibitory output neurons of the preoptic area. The resulting disinhibition of thermogenesis-promoting neurons in the dorsomedial hypothalamus and possibly of sympathetic and somatic premotor neurons in the rostral ventromedial medulla, including the raphe pallidus, activates excitatory inputs to spinal sympathetic and somatic motor circuits to drive thermogenesis.
Figures

Similar articles
-
Central control of brown adipose tissue thermogenesis.Front Endocrinol (Lausanne). 2012 Jan 24;3(5):5. doi: 10.3389/fendo.2012.00005. Front Endocrinol (Lausanne). 2012. PMID: 22389645 Free PMC article.
-
Central circuitries for body temperature regulation and fever.Am J Physiol Regul Integr Comp Physiol. 2011 Nov;301(5):R1207-28. doi: 10.1152/ajpregu.00109.2011. Epub 2011 Sep 7. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21900642 Review.
-
Central neural control of thermoregulation and brown adipose tissue.Auton Neurosci. 2016 Apr;196:14-24. doi: 10.1016/j.autneu.2016.02.010. Epub 2016 Feb 23. Auton Neurosci. 2016. PMID: 26924538 Free PMC article. Review.
-
Central neural pathways for thermoregulation.Front Biosci (Landmark Ed). 2011 Jan 1;16(1):74-104. doi: 10.2741/3677. Front Biosci (Landmark Ed). 2011. PMID: 21196160 Free PMC article. Review.
-
2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense.J Appl Physiol (1985). 2011 May;110(5):1137-49. doi: 10.1152/japplphysiol.01227.2010. Epub 2011 Jan 26. J Appl Physiol (1985). 2011. PMID: 21270352 Free PMC article.
Cited by
-
Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters.Am J Physiol Regul Integr Comp Physiol. 2017 Mar 1;312(3):R324-R337. doi: 10.1152/ajpregu.00456.2015. Epub 2017 Jan 11. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28077392 Free PMC article.
-
Mechanism of H₂ histamine receptor dependent modulation of body temperature and neuronal activity in the medial preoptic nucleus.Neuropharmacology. 2012 Aug;63(2):171-80. doi: 10.1016/j.neuropharm.2012.02.006. Epub 2012 Feb 16. Neuropharmacology. 2012. PMID: 22366077 Free PMC article.
-
PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation.Am J Physiol Regul Integr Comp Physiol. 2015 Oct 15;309(8):R795-804. doi: 10.1152/ajpregu.00333.2015. Epub 2015 Aug 19. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26290108 Free PMC article. Review.
-
Insomnia Caused by Serotonin Depletion is Due to Hypothermia.Sleep. 2015 Dec 1;38(12):1985-93. doi: 10.5665/sleep.5256. Sleep. 2015. PMID: 26194567 Free PMC article.
-
COVID-19 risks and systemic gaps in Nigeria: resilience building lessons for pandemic and climate change management.SN Soc Sci. 2022;2(11):247. doi: 10.1007/s43545-022-00557-8. Epub 2022 Oct 30. SN Soc Sci. 2022. PMID: 36339526 Free PMC article.
References
-
- Aicher SA, Hahn B, Milner TA. N-Methyl-d-aspartate-type glutamate receptors are found in post-synaptic targets of adrenergic terminals in the thoracic spinal cord. Brain Research. 2000;856:1–11. - PubMed
-
- Bacon SJ, Smith AD. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst. 1988;24:97–122. - PubMed
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1569–R1578. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

