Random-walk model of diffusion in three dimensions in brain extracellular space: comparison with microfiberoptic photobleaching measurements
- PMID: 18469079
- PMCID: PMC2483768
- DOI: 10.1529/biophysj.108.131466
Random-walk model of diffusion in three dimensions in brain extracellular space: comparison with microfiberoptic photobleaching measurements
Abstract
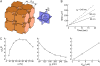
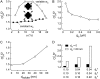
Diffusion through the extracellular space (ECS) in brain is important in drug delivery, intercellular communication, and extracellular ionic buffering. The ECS comprises approximately 20% of brain parenchymal volume and contains cell-cell gaps approximately 50 nm. We developed a random-walk model to simulate macromolecule diffusion in brain ECS in three dimensions using realistic ECS dimensions. Model inputs included ECS volume fraction (alpha), cell size, cell-cell gap geometry, intercellular lake (expanded regions of brain ECS) dimensions, and molecular size of the diffusing solute. Model output was relative solute diffusion in water versus brain ECS (D(o)/D). Experimental D(o)/D for comparison with model predictions was measured using a microfiberoptic fluorescence photobleaching method involving stereotaxic insertion of a micron-size optical fiber into mouse brain. D(o)/D for the small solute calcein in different regions of brain was in the range 3.0-4.1, and increased with brain cell swelling after water intoxication. D(o)/D also increased with increasing size of the diffusing solute, particularly in deep brain nuclei. Simulations of measured D(o)/D using realistic alpha, cell size and cell-cell gap required the presence of intercellular lakes at multicell contact points, and the contact length of cell-cell gaps to be least 50-fold smaller than cell size. The model accurately predicted D(o)/D for different solute sizes. Also, the modeling showed unanticipated effects on D(o)/D of changing ECS and cell dimensions that implicated solute trapping by lakes. Our model establishes the geometric constraints to account quantitatively for the relatively modest slowing of solute and macromolecule diffusion in brain ECS.
Figures




References
-
- Mazel, T., Z. Simonova, and E. Sykova. 1998. Diffusion heterogeneity and anisotropy in rat hippocampus. Neuroreport. 9:1299–1304. - PubMed
-
- Nicholson, C., and E. Sykova. 1998. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21:207–215. - PubMed
-
- Nicholson, C., K. C. Chen, S. Hrabetova, and L. Tao. 2000. Diffusion of molecules in brain extracellular space: theory and experiment. Prog. Brain Res. 125:129–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

