PrP(106-126) does not interact with membranes under physiological conditions
- PMID: 18469080
- PMCID: PMC2483773
- DOI: 10.1529/biophysj.108.131458
PrP(106-126) does not interact with membranes under physiological conditions
Abstract
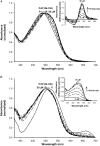
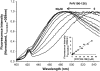
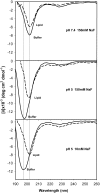
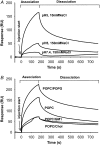
Transmissible spongiform encephalopathies are neurodegenerative diseases characterized by the accumulation of an abnormal isoform of the prion protein PrP(Sc). Its fragment 106-126 has been reported to maintain most of the pathological features of PrP(Sc), and a role in neurodegeneration has been proposed based on the modulation of membrane properties and channel formation. The ability of PrP(Sc) to modulate membranes and/or form channels in membranes has not been clearly demonstrated; however, if these processes are important, peptide-membrane interactions would be a key feature in the toxicity of PrP(Sc). In this work, the interaction of PrP(106-126) with model membranes comprising typical lipid identities, as well as more specialized lipids such as phosphatidylserine and GM1 ganglioside, was examined using surface plasmon resonance and fluorescence methodologies. This comprehensive study examines different parameters relevant to characterization of peptide-membrane interactions, including membrane charge, viscosity, lipid composition, pH, and ionic strength. We report that PrP(106-126) has a low affinity for lipid membranes under physiological conditions without evidence of membrane disturbances. Membrane insertion and leakage occur only under conditions in which strong electrostatic interactions operate. These results support the hypothesis that the physiological prion protein PrP(C) mediates PrP(106-126) toxic effects in neuronal cells.
Figures



Similar articles
-
The role of prion peptide structure and aggregation in toxicity and membrane binding.J Neurochem. 2000 Dec;75(6):2536-45. doi: 10.1046/j.1471-4159.2000.0752536.x. J Neurochem. 2000. PMID: 11080207
-
The toxicity of prion protein fragment PrP(106-126) is not mediated by membrane permeabilization as shown by a M112W substitution.Biochemistry. 2009 May 19;48(19):4198-208. doi: 10.1021/bi900009d. Biochemistry. 2009. PMID: 19301918
-
A neurotoxic and gliotrophic fragment of the prion protein increases plasma membrane microviscosity.Neurobiol Dis. 1997;4(1):47-57. doi: 10.1006/nbdi.1997.0133. Neurobiol Dis. 1997. PMID: 9258911
-
Is PrP(106-126) fragment involved in the membrane activity of the Prion protein?Curr Protein Pept Sci. 2010 Aug;11(5):326-33. doi: 10.2174/138920310791330604. Curr Protein Pept Sci. 2010. PMID: 20423298 Review.
-
Mechanisms of prion-induced modifications in membrane transport properties: implications for signal transduction and neurotoxicity.Chem Biol Interact. 2001 Oct 25;138(1):1-26. doi: 10.1016/s0009-2797(01)00228-9. Chem Biol Interact. 2001. PMID: 11640912 Review.
Cited by
-
Phosphatidylethanolamine binding is a conserved feature of cyclotide-membrane interactions.J Biol Chem. 2012 Sep 28;287(40):33629-43. doi: 10.1074/jbc.M112.372011. Epub 2012 Aug 1. J Biol Chem. 2012. PMID: 22854971 Free PMC article.
-
The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation.J Neuroinflammation. 2012 Jul 11;9:73. doi: 10.1186/1742-2094-9-73. J Neuroinflammation. 2012. PMID: 22531291 Free PMC article.
-
Decoding the membrane activity of the cyclotide kalata B1: the importance of phosphatidylethanolamine phospholipids and lipid organization on hemolytic and anti-HIV activities.J Biol Chem. 2011 Jul 8;286(27):24231-41. doi: 10.1074/jbc.M111.253393. Epub 2011 May 16. J Biol Chem. 2011. PMID: 21576247 Free PMC article.
-
Bacteria May Cope Differently from Similar Membrane Damage Caused by the Australian Tree Frog Antimicrobial Peptide Maculatin 1.1.J Biol Chem. 2015 Aug 7;290(32):19853-62. doi: 10.1074/jbc.M115.643262. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100634 Free PMC article.
-
Mechanisms of bacterial membrane permeabilization by crotalicidin (Ctn) and its fragment Ctn(15-34), antimicrobial peptides from rattlesnake venom.J Biol Chem. 2018 Feb 2;293(5):1536-1549. doi: 10.1074/jbc.RA117.000125. Epub 2017 Dec 18. J Biol Chem. 2018. PMID: 29255091 Free PMC article.
References
-
- Johnson, R. T. 2005. Prion diseases. Lancet Neurol. 4:635–642. - PubMed
-
- Naslavsky, N., H. Shmeeda, G. Friedlander, A. Yanai, A. H. Futerman, Y. Barenholz, and A. Taraboulos. 1999. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J. Biol. Chem. 274:20763–20771. - PubMed
-
- Pinheiro, T. J. 2006. The role of rafts in the fibrillization and aggregation of prions. Chem. Phys. Lipids. 141:66–71. - PubMed
-
- Thellung, S., T. Florio, A. Corsaro, S. Arena, M. Merlino, M. Salmona, F. Tagliavini, O. Bugiani, G. Forloni, and G. Schettini. 2000. Intracellular mechanisms mediating the neuronal death and astrogliosis induced by the prion protein fragment 106-126. Int. J. Dev. Neurosci. 18:481–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

