Kinetics, statistics, and energetics of lipid membrane electroporation studied by molecular dynamics simulations
- PMID: 18469089
- PMCID: PMC2483751
- DOI: 10.1529/biophysj.108.129437
Kinetics, statistics, and energetics of lipid membrane electroporation studied by molecular dynamics simulations
Abstract
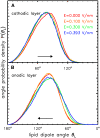
Membrane electroporation is the method to directly transfer bioactive substances such as drugs and genes into living cells, as well as preceding electrofusion. Although much information on the microscopic mechanism has been obtained both from experiment and simulation, the existence and nature of possible intermediates is still unclear. To elucidate intermediates of electropore formation by direct comparison with measured prepore formation kinetics, we have carried out 49 atomistic electroporation simulations on a palmitoyl-oleoyl-phosphatidylcholine bilayer for electric field strengths between 0.04 and 0.7 V/nm. A statistical theory is developed to facilitate direct comparison of experimental (macroscopic) prepore formation kinetics with the (single event) preporation times derived from the simulations, which also allows us to extract an effective number of lipids involved in each pore formation event. A linear dependency of the activation energy for prepore formation on the applied field is seen, with quantitative agreement between experiment and simulation. The distribution of preporation times suggests a four-state pore formation model. The model involves a first intermediate characterized by a differential tilt of the polar lipid headgroups on both leaflets, and a second intermediate (prepore), where a polar chain across the bilayer is formed by 3-4 lipid headgroups and several water molecules, thereby providing a microscopic explanation for the polarizable volume derived previously from the measured kinetics. An average pore radius of 0.47 +/- 0.15 nm is seen, in favorable agreement with conductance measurements and electrooptical experiments of lipid vesicles.
Figures











References
-
- Sale, A. J. H., and W. A. Hamilton. 1967. Effects of high electric fields on microorganisms. I. Killing of bacteria and yeast. Biochim. Biophys. Acta. 148:781–788.
-
- Neumann, E., and K. Rosenheck. 1972. Permeability changes induced by electric impulses in vesicular membranes. J. Mol. Biol. 10:279–290. - PubMed
-
- Belehradek, M., C. Domenge, B. Luboinski, S. Orlowski, J. Belehradek, and L. M. Mir. 1993. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 72:3694–3700. - PubMed
-
- Mir, L. M., S. Orlowski, J. Belehradek, J. Teissie, M. P. Rols, G. Sersa, D. Miklavcic, R. Gilbert, and R. Heller. 1995. Biomedical applications of electric pulses with special emphasis on antitumor electrochemotherapy. Bioelectrochem. Bioenerg. 38:203–207.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

