The CI repressors of Shiga toxin-converting prophages are involved in coinfection of Escherichia coli strains, which causes a down regulation in the production of Shiga toxin 2
- PMID: 18469095
- PMCID: PMC2446792
- DOI: 10.1128/JB.00069-08
The CI repressors of Shiga toxin-converting prophages are involved in coinfection of Escherichia coli strains, which causes a down regulation in the production of Shiga toxin 2
Abstract
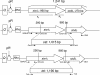
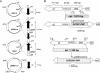
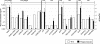
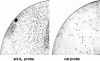
Shiga toxins (Stx) are the main virulence factors associated with a form of Escherichia coli known as Shiga toxin-producing E. coli (STEC). They are encoded in temperate lambdoid phages located on the chromosome of STEC. STEC strains can carry more than one prophage. Consequently, toxin and phage production might be influenced by the presence of more than one Stx prophage on the bacterial chromosome. To examine the effect of the number of prophages on Stx production, we produced E. coli K-12 strains carrying either one Stx2 prophage or two different Stx2 prophages. We used recombinant phages in which an antibiotic resistance gene (aph, cat, or tet) was incorporated in the middle of the Shiga toxin operon. Shiga toxin was quantified by immunoassay and by cytotoxicity assay on Vero cells (50% cytotoxic dose). When two prophages were inserted in the host chromosome, Shiga toxin production and the rate of lytic cycle activation fell. The cI repressor seems to be involved in incorporation of the second prophage. Incorporation and establishment of the lysogenic state of the two prophages, which lowers toxin production, could be regulated by the CI repressors of both prophages operating in trans. Although the sequences of the cI genes of the phages studied differed, the CI protein conformation was conserved. Results indicate that the presence of more than one prophage in the host chromosome could be regarded as a mechanism to allow genetic retention in the cell, by reducing the activation of lytic cycle and hence the pathogenicity of the strains.
Figures



Similar articles
-
Differential induction of Shiga toxin in environmental Escherichia coli O145:H28 strains carrying the same genotype as the outbreak strains.Int J Food Microbiol. 2021 Feb 2;339:109029. doi: 10.1016/j.ijfoodmicro.2020.109029. Epub 2020 Dec 23. Int J Food Microbiol. 2021. PMID: 33360585
-
Prophages integrating into prophages: A mechanism to accumulate type III secretion effector genes and duplicate Shiga toxin-encoding prophages in Escherichia coli.PLoS Pathog. 2021 Apr 29;17(4):e1009073. doi: 10.1371/journal.ppat.1009073. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33914852 Free PMC article.
-
Induction of Shiga Toxin-Encoding Prophage by Abiotic Environmental Stress in Food.Appl Environ Microbiol. 2017 Sep 15;83(19):e01378-17. doi: 10.1128/AEM.01378-17. Print 2017 Oct 1. Appl Environ Microbiol. 2017. PMID: 28778890 Free PMC article.
-
Altruism of Shiga toxin-producing Escherichia coli: recent hypothesis versus experimental results.Front Cell Infect Microbiol. 2013 Jan 4;2:166. doi: 10.3389/fcimb.2012.00166. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316482 Free PMC article. Review.
-
Bacteriophages of Shiga Toxin-Producing Escherichia coli and Their Contribution to Pathogenicity.Pathogens. 2021 Mar 29;10(4):404. doi: 10.3390/pathogens10040404. Pathogens. 2021. PMID: 33805526 Free PMC article. Review.
Cited by
-
Variability in the Occupancy of Escherichia coli O157 Integration Sites by Shiga Toxin-Encoding Prophages.Toxins (Basel). 2021 Jun 22;13(7):433. doi: 10.3390/toxins13070433. Toxins (Basel). 2021. PMID: 34206386 Free PMC article.
-
ppGpp and cytotoxicity diversity in Shiga toxin-producing Escherichia coli (STEC) isolates.Epidemiol Infect. 2017 Aug;145(11):2204-2211. doi: 10.1017/S0950268817001091. Epub 2017 Jun 7. Epidemiol Infect. 2017. PMID: 28587697 Free PMC article.
-
Core and Accessory Genome Comparison of Australian and International Strains of O157 Shiga Toxin-Producing Escherichia coli.Front Microbiol. 2020 Sep 4;11:566415. doi: 10.3389/fmicb.2020.566415. eCollection 2020. Front Microbiol. 2020. PMID: 33013798 Free PMC article.
-
The Prophage and Us-Shiga Toxin Phages Revisited.Pathogens. 2023 Feb 2;12(2):232. doi: 10.3390/pathogens12020232. Pathogens. 2023. PMID: 36839504 Free PMC article.
-
The Virulence of Escherichia coli O157:H7 Isolates in Mice Depends on Shiga Toxin Type 2a (Stx2a)-Induction and High Levels of Stx2a in Stool.Front Cell Infect Microbiol. 2020 Feb 26;10:62. doi: 10.3389/fcimb.2020.00062. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32175286 Free PMC article.
References
-
- Allison, H. E., M. J. Sergeant, C. E. James, J. R. Saunders, D. L. Smith, R. J. Sharp, T. S. Marks, and A. J. McCarthy. 2003. Immunity profiles of wild-type and recombinant Shiga-like toxin-encoding bacteriophages and characterization of novel double lysogens. Infect. Immun. 713409-3418. - PMC - PubMed
-
- Bielaszewska, M., W. Zhang, A. Mellmann, and H. Karch. 2007. Enterohaemorrhagic Escherichia coli O26:H11/H-: a human pathogen in emergence. Berl. Munch. Tierarztl. Wochenschr. 120279-287. - PubMed
-
- Blanco, J., M. Blanco, J. E. Blanco, A. Mora, M. P. Alonso, E. A. González, and M. I. Bernárdez. 2001. Epidemiology of verocytotoxigenic Escherichia coli (VTEC) in ruminants, p. 113-148. In G. Duffy, P. Garvey, and D. McDowell (ed.), Verocytotoxigenic Escherichia coli. Food & Nutrition Press Inc., Trumbull, CT.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

