Substrate specificity of the 3-methylcrotonyl coenzyme A (CoA) and geranyl-CoA carboxylases from Pseudomonas aeruginosa
- PMID: 18469096
- PMCID: PMC2447018
- DOI: 10.1128/JB.00454-08
Substrate specificity of the 3-methylcrotonyl coenzyme A (CoA) and geranyl-CoA carboxylases from Pseudomonas aeruginosa
Abstract
Biotin-containing 3-methylcrotonyl coenzyme A (MC-CoA) carboxylase (MCCase) and geranyl-CoA (G-CoA) carboxylase (GCCase) from Pseudomonas aeruginosa were expressed as His-tagged recombinant proteins in Escherichia coli. Both native and recombinant MCCase and GCCase showed pH and temperature optima of 8.5 and 37 degrees C. The apparent K(0.5) (affinity constant for non-Michaelis-Menten kinetics behavior) values of MCCase for MC-CoA, ATP, and bicarbonate were 9.8 microM, 13 microM, and 0.8 microM, respectively. MCCase activity showed sigmoidal kinetics for all the substrates and did not carboxylate G-CoA. In contrast, GCCase catalyzed the carboxylation of both G-CoA and MC-CoA. GCCase also showed sigmoidal kinetic behavior for G-CoA and bicarbonate but showed Michaelis-Menten kinetics for MC-CoA and the cosubstrate ATP. The apparent K(0.5) values of GCCase were 8.8 microM and 1.2 microM for G-CoA and bicarbonate, respectively, and the apparent K(m) values of GCCase were 10 microM for ATP and 14 microM for MC-CoA. The catalytic efficiencies of GCCase for G-CoA and MC-CoA were 56 and 22, respectively, indicating that G-CoA is preferred over MC-CoA as a substrate. The enzymatic properties of GCCase suggest that it may substitute for MCCase in leucine catabolism and that both the MCCase and GCCase enzymes play important roles in the leucine and acyclic terpene catabolic pathways.
Figures

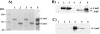

References
-
- Chen, Y., E. S. Wurtele, X. Wang, and B. J. Nikolau. 1993. Purification and characterization of 3-methylcrotonyl-CoA carboxylase from somatic embryos of Daucus carota. Arch. Biochem. Biophys. 305103-109. - PubMed
-
- Diacovich, L., S. Peiru, D. Kurth, E. Rodriguez, F. Podesta, C. Khosla, and H. Gramajo. 2002. Kinetic and structural analysis of the new group of acyl-CoA carboxylases found in Streptomyces coelicolor A3(2). J. Biol. Chem. 27731228-31236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

