Functional analysis of GlnE, an essential adenylyl transferase in Mycobacterium tuberculosis
- PMID: 18469098
- PMCID: PMC2446997
- DOI: 10.1128/JB.00166-08
Functional analysis of GlnE, an essential adenylyl transferase in Mycobacterium tuberculosis
Abstract
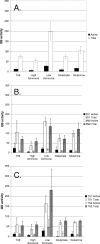
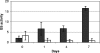
Glutamine synthetase (GS) plays an important role in nitrogen assimilation. The major GS of Mycobacterium tuberculosis is GlnA1, a type I GS whose activity is controlled by posttranscriptional modification by GlnE. GlnE is an adenylyl transferase comprised of an adenylylating domain and a deadenylylating domain which modulate GS activity. We previously demonstrated that GlnE is essential in M. tuberculosis in normal growth medium. In this study, we further show that GlnE is required under multiple medium conditions, including in nitrogen-limited medium. We demonstrate that adenylylation is the critical activity for M. tuberculosis survival, since we were able to delete the deadenylylation domain with no apparent effect on growth or GS activity. Furthermore, we identified a critical aspartate residue in the proposed nucleotidyltransferase motif. Temperature-sensitive mutants of GlnE were generated and shown to have a defect in growth and GS activity in nitrogen-limited medium. Finally, we were able to generate a GlnE null mutant in the presence of L-methionine sulfoximine, a GS inhibitor, and glutamine supplementation. In the presence of these supplements, the null mutant was able to grow similarly to the wild type. Surprisingly, the GlnE mutant was able to survive and grow for extended periods in liquid medium, but not on solid medium, in the absence of GS inhibition. Thus, we have confirmed that the unusual requirement of M. tuberculosis for GlnE adenylylation activity is linked to the activity of GS in the cell.
Figures



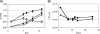
References
-
- Almassy, R. J., C. A. Janson, R. Hamlin, N. H. Xuong, and D. Eisenberg. 1986. Novel subunit-subunit interactions in the structure of glutamine synthetase. Nature 323304-309. - PubMed
-
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, 3rd, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. - PubMed
-
- Collins, D. M., T. Wilson, S. Campbell, B. M. Buddle, B. J. Wards, G. Hotter, and G. W. De Lisle. 2002. Production of avirulent mutants of Mycobacterium bovis with vaccine properties by the use of illegitimate recombination and screening of stationary-phase cultures. Microbiology 1483019-3027. - PubMed
-
- Downing, K. J., R. A. McAdam, and V. Mizrahi. 1999. Staphylococcus aureus nuclease is a useful secretion reporter for mycobacteria. Gene 239293-299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

