Identification and quantitation of cobalamin and cobalamin analogues in human feces
- PMID: 18469256
- PMCID: PMC2900183
- DOI: 10.1093/ajcn/87.5.1324
Identification and quantitation of cobalamin and cobalamin analogues in human feces
Abstract
Background: Cobalamin (vitamin B-12) and cobalamin analogues are present in human feces, but a complete identification has not been established, and the amounts present have not been determined.
Objectives: We aimed to develop a liquid chromatography-mass spectrometry method for cobalamin and cobalamin analogues and to identify and quantitiate the amounts present in human feces.
Design: Fecal samples were obtained from 20 human subjects in good general health. The samples were analyzed for the presence and amounts of cobalamin and 12 cobalamin analogues that were synthesized with and without the incorporation of stable isotopes.
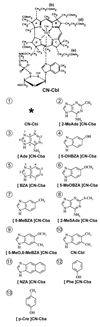
Results: Cobalamin and 7 cobalamin analogues were identified and quantitated in human feces. The mean for the total amount present in 18 subjects whose daily intake was < or = 25 microg cobalamin from vitamin supplements was 1309 ng cobalamin equivalents/g wet wt of feces. Cobalamin (1.4%) and cobinamide (1.8%) (an incomplete corrinoid) represented a small portion of the total amount. Six cobalamin analogues that contain a base other than the 5,6-dimethylbenzimidizidole in cobalamin were present. The bases and their mean amounts (in %) are 2-methyladenine (60.6%), p-cresol (16.3%), adenine (12.5%), 2-(methylthio)adenine (15.5%), 5-hydroxybenzimidazole (1.8%), and phenol (0.1%). One subject ingested 1 mg cobalamin/d and another ingested 2 mg cobalamin/d, and they appeared to convert most of the cobalamin to cobinamide and the 4 analogues that contain the bases-2-methyladenine, p-cresol, adenine, and 2-(methylthio)adenine.
Conclusions: Cobalamin analogues are present in human feces and account for > 98% of the total of cobalamin plus cobalamin analogues. A major portion of large amounts of ingested cobalamin appears to be converted to cobalamin analogues.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Renz P. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids. In: Banerjee R, editor. Chemistry and Biochemistry of B12. 1st ed. New York: John Wiley & Sons, Inc; 1999. pp. 557–575.
-
- Allen Robert H. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. - PubMed
-
- Kolhouse JF, Allen RH. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977;60:1381–1392. - PMC - PubMed
-
- Seetharam B, Yammani RR. Cobalamin transport proteins and their cell-surface receptors. Expert Rev Mol Med. 2003;5:1–18. - PubMed
-
- Stupperich E, Nexo E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur J Biochem. 1991;199:299–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

