Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose
- PMID: 18469330
- PMCID: PMC2734297
- DOI: 10.1093/toxsci/kfn091
Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose
Abstract
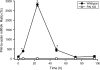
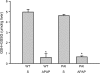
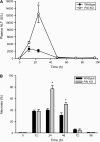
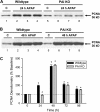
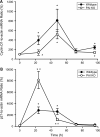
Deficiency in plasminogen activator inhibitor-1 (PAI-1) gene expression is known to promote growth factor activation and regeneration in a number of hepatotoxicity models. To evaluate if PAI-1 has similar effects in acetaminophen (APAP) hepatotoxicity, wild-type (WT) and PAI-1 gene knockout mice (PAI-KO) were treated with 200 mg/kg APAP and liver injury and its repair were assessed. In WT animals, plasma alanine aminotransferase (ALT) activities increased during the first 12 h and then returned to baseline within 48 h. The area of necrosis increased in parallel to the ALT values, peaked between 12 and 24 h and was completely resolved by 96 h. The regenerative response of cells outside the necrotic area, as indicated by proliferating cell nuclear antigen protein and cyclin D(1) gene expression, was observed within 24 h, peaked at 48 h and then declined but remained elevated until 96 h. Liver injury in response to APAP was similar in PAI-KO as in WT animals during the first 12 h. However, plasma ALT values and the area of necrosis further increased during the following 12 h with development of massive intrahepatic hemorrhage. Approximately, 50% of the PAI-KO animals did not survive. Although liver injury of the surviving animals was repaired, the regeneration process was delayed until 48 h. A potential reason for this delay may have been due to the more severe injury and/or the increased expression of the cell cycle inhibitor p21. Our data indicate that PAI activation limits liver injury and mortality during APAP hepatotoxicity by preventing excessive hemorrhage and thereby facilitating tissue repair.
Figures



References
-
- Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397–404. - PubMed
-
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver injury. Toxicol. Sci. 2006;94:217–225. - PubMed
-
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. - PubMed
-
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J. Pharmacol. Exp. Ther. 2003;307:67–73. - PubMed
-
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: Evidence for postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

