Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases
- PMID: 18470571
- PMCID: PMC2467497
- DOI: 10.1245/s10434-008-9881-6
Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases
Abstract
Purpose: The liver is one of the most common sites for metastatic solid tumors. If the liver is the only site of metastatic disease, regional treatment options can offer the benefit of high local exposure with limited systemic toxicity, especially for patients without (further) systemic treatment options. We report the results of our experience with isolated hepatic perfusion (IHP) in patients with isolated liver metastases from a variety of primary tumors.
Patients and methods: Nineteen patients with isolated unresectable liver metastases from a variety of tumors (13 uveal melanomas, 2 neuroendocrine carcinomas, 2 gastrointestinal stromal tumors, 1 hepatocellular carcinoma, and 1 high-grade sarcoma) were treated with a 60-min IHP using 200 mg melphalan. Patients were monitored for toxicity, response according to response evaluation criteria in solid tumors (RECIST) criteria, and survival.
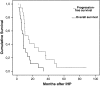
Results: One melanoma patient was not perfused due to insufficient isolation of the liver. There was no treatment-related mortality. Reversible grade 3 or 4 hepatoxicity occurred in 10 (56%) patients, while veno-occlusive disease occurred in 4 (22%) patients. Of the 12 uveal melanoma patients who were perfused, 4 (33%) patients had a partial hepatic response, 6 (50%) patients had stable hepatic disease, and 2 (17%) patients were immediately progressive. Median disease-free survival was 6.6 months with a median overall survival of 10.0 months. Fifty percent of other primary tumors showed at least partial remission, including one complete remission in a high-grade sarcoma patient.
Conclusion: IHP with melphalan shows activity in patients with liver metastases from a variety of primary tumors, but other or additional drugs may improve therapeutic outcome.
Figures
Similar articles
-
Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver.Melanoma Res. 2004 Feb;14(1):67-72. doi: 10.1097/00008390-200402000-00011. Melanoma Res. 2004. PMID: 15091197 Clinical Trial.
-
Survival and Quality of Life After Isolated Hepatic Perfusion With Melphalan as a Treatment for Uveal Melanoma Liver Metastases: Final Results From the Phase III Randomized Controlled Trial SCANDIUM.Ann Surg. 2025 Jul 1;282(1):100-107. doi: 10.1097/SLA.0000000000006255. Epub 2024 Feb 29. Ann Surg. 2025. PMID: 38420778 Free PMC article. Clinical Trial.
-
A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver.Clin Cancer Res. 2000 Aug;6(8):3062-70. Clin Cancer Res. 2000. PMID: 10955785 Clinical Trial.
-
Optimizing the treatment of liver metastases from uveal melanomas with transarterial chemoembolization using melphalan and calibrated microspheres.Bull Cancer. 2020 Dec;107(12):1274-1283. doi: 10.1016/j.bulcan.2020.09.010. Epub 2020 Nov 9. Bull Cancer. 2020. PMID: 33183739 Review.
-
The past decade of experience with isolated hepatic perfusion.Oncologist. 2004;9(6):653-64. doi: 10.1634/theoncologist.9-6-653. Oncologist. 2004. PMID: 15561809 Review.
Cited by
-
Hepatic artery infusion of melphalan in patients with liver metastases from ocular melanoma.J Surg Oncol. 2018 Apr;117(5):940-946. doi: 10.1002/jso.24984. J Surg Oncol. 2018. PMID: 29878390 Free PMC article.
-
New Therapeutic Perspectives in the Treatment of Uveal Melanoma: A Systematic Review.Biomedicines. 2021 Sep 24;9(10):1311. doi: 10.3390/biomedicines9101311. Biomedicines. 2021. PMID: 34680428 Free PMC article. Review.
-
Isolated hepatic perfusion for patients with liver metastases.Ther Adv Med Oncol. 2014 Jul;6(4):180-94. doi: 10.1177/1758834014529175. Ther Adv Med Oncol. 2014. PMID: 25057304 Free PMC article. Review.
-
Percutaneous isolated liver perfusion with occlusion balloons and a catheter-based stent-graft-like perfusion device: an experimental study in a porcine model.Eur Radiol. 2010 Oct;20(10):2372-80. doi: 10.1007/s00330-010-1816-5. Epub 2010 May 22. Eur Radiol. 2010. PMID: 20495978
-
Regional chemotherapy for uveal melanoma liver metastases.Int J Ophthalmol. 2023 Feb 18;16(2):293-300. doi: 10.18240/ijo.2023.02.18. eCollection 2023. Int J Ophthalmol. 2023. PMID: 36816216 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical