Hinge stiffness is a barrier to RNA folding
- PMID: 18471829
- PMCID: PMC2518058
- DOI: 10.1016/j.jmb.2008.04.013
Hinge stiffness is a barrier to RNA folding
Abstract
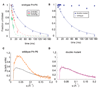
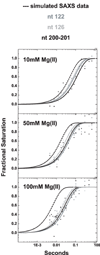
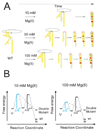
Cation-mediated RNA folding from extended to compact, biologically active conformations relies on a temporal balance of forces. The Mg2 +-mediated folding of the Tetrahymena thermophila ribozyme is characterized by rapid nonspecific collapse followed by tertiary-contact-induced compaction. This article focuses on an autonomously folding portion of the Tetrahymena ribozyme, its P4-P6 domain, in order to probe one facet of the rapid collapse: chain flexibility. The time evolution of P4-P6 folding was followed by global and local measures as a function of Mg2 + concentration. While all concentrations of Mg2 + studied are sufficient to screen the charge on the helices, the rates of compaction and tertiary contact formation diverge as the concentration of Mg2 + increases; collapse is greatly accelerated by Mg2 +, while tertiary contact formation is not. These studies highlight the importance of chain stiffness to RNA folding; at 10 mM Mg2 +, a stiff hinge limits the rate of P4-P6 folding. At higher magnesium concentrations, the rate-limiting step shifts from hinge bending to tertiary contact formati
Figures


References
-
- Crothers DM, Cole PE, Hilbers CW, Shulman RG. Molecular Mechanism of Thermal Unfolding of Escherichia-Coli Formylmethionine Transfer-RNA. J. Mol. Biol. 1974;87:63–72. - PubMed
-
- Duckett DR, Murchie AIH, Clegg RM, Bassi GS, Giraud-Panis MJE, Lilley DMJ. Nucleic acid structure and recognition. Biophys. Chem. 1997;68:53–62. - PubMed
-
- Hagerman PJ. Flexibility of RNA. Annual Review of Biophysics and Biomolecular Structure. 1997;26:139–156. - PubMed
-
- Takamoto K, Das R, He Q, Doniach S, Brenowitz M, Herschlag D, Chance MR. Principles of RNA compaction: Insights from the equilibrium folding pathway of the P4–P6 RNA domain in monovalent cations. J. Mol. Biol. 2004;343:1195–1206. - PubMed
-
- Takamoto K, He Q, Morris S, Chance MR, Brenowitz M. Monovalent cations mediate formation of native tertiary structure of the Tetrahymena thermophila ribozyme. Nat. Struct. Biol. 2002;9:928–933. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

