A survey of acute and chronic heroin dependence in ten inbred mouse strains: evidence of genetic correlation with morphine dependence
- PMID: 18472145
- PMCID: PMC3627368
- DOI: 10.1016/j.pbb.2008.03.030
A survey of acute and chronic heroin dependence in ten inbred mouse strains: evidence of genetic correlation with morphine dependence
Abstract
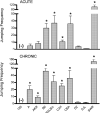
Heroin and morphine exposure can cause physical dependence, with symptoms manifesting during their withdrawal. Inter-individual differences in symptom frequency during morphine withdrawal are a common finding that, in rodents, is demonstrably attributable to genotype. However, it is not known whether inter-individual differences characterize heroin withdrawal, and whether such variation can be similarly influenced by genotype. Therefore, we injected mice of ten inbred strains with acute and chronic heroin doses and compared their jumping frequencies, a common index of withdrawal magnitude, during naloxone-precipitated withdrawal. The data revealed significant strain frequency differences (range after acute and chronic heroin injection: 0-104 and 0-142 jumps, respectively) and substantial heritability (h(2)=0.94 to 0.96), indicating that genetic variance is associated with heroin withdrawal. The rank order of strain sensitivity for acute and chronic heroin withdrawal jumping, and for the current heroin and previous morphine strain data, were significantly correlated (r=0.75-0.94), indicating their genetic and, ultimately, physiological commonality. These data suggest that the genetic liability to heroin dependence remains constant across a period of heroin intake, and that heroin and morphine dependence may benefit from common treatment strategies.
Figures

Similar articles
-
Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence.Neuroscience. 2002;115(2):463-9. doi: 10.1016/s0306-4522(02)00458-x. Neuroscience. 2002. PMID: 12421612
-
Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors.Eur J Pharmacol. 2008 May 31;586(1-3):179-88. doi: 10.1016/j.ejphar.2008.02.035. Epub 2008 Feb 19. Eur J Pharmacol. 2008. PMID: 18343363 Free PMC article.
-
The contribution of MOR-1 exons 1-4 to morphine and heroin analgesia and dependence.Neurosci Lett. 2009 Jul 3;457(3):115-9. doi: 10.1016/j.neulet.2009.04.012. Epub 2009 Apr 9. Neurosci Lett. 2009. PMID: 19429175
-
Gnao1 (G alphaO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice.Neuroscience. 2009 Sep 15;162(4):1255-64. doi: 10.1016/j.neuroscience.2009.05.027. Epub 2009 May 19. Neuroscience. 2009. PMID: 19460419
-
Physiological dependence to mitragynine indicated by a rapid cross-dependence procedure with heroin-dependent mice.Psychopharmacology (Berl). 2022 Mar;239(3):897-908. doi: 10.1007/s00213-022-06080-1. Epub 2022 Feb 2. Psychopharmacology (Berl). 2022. PMID: 35107609
Cited by
-
A history of chronic morphine exposure during adolescence increases despair-like behaviour and strain-dependently promotes sociability in abstinent adult mice.Behav Brain Res. 2013 Apr 15;243:44-52. doi: 10.1016/j.bbr.2012.12.049. Epub 2013 Jan 4. Behav Brain Res. 2013. PMID: 23295400 Free PMC article.
-
Functional genomic mechanisms of opioid action and opioid use disorder: a systematic review of animal models and human studies.Mol Psychiatry. 2023 Nov;28(11):4568-4584. doi: 10.1038/s41380-023-02238-1. Epub 2023 Sep 15. Mol Psychiatry. 2023. PMID: 37723284 Free PMC article.
-
Motivational effects of opiates in conditioned place preference and aversion paradigm--a study in three inbred strains of mice.Psychopharmacology (Berl). 2009 Dec;207(2):245-55. doi: 10.1007/s00213-009-1672-7. Epub 2009 Sep 29. Psychopharmacology (Berl). 2009. PMID: 19787337
-
Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence.Neuropsychopharmacology. 2014 Oct;39(11):2694-705. doi: 10.1038/npp.2014.126. Epub 2014 May 30. Neuropsychopharmacology. 2014. PMID: 24874714 Free PMC article.
-
Dissociation of heroin-induced emotional dysfunction from psychomotor activation and physical dependence among inbred mouse strains.Psychopharmacology (Berl). 2015 Jun;232(11):1957-71. doi: 10.1007/s00213-014-3826-5. Epub 2014 Dec 9. Psychopharmacology (Berl). 2015. PMID: 25482274
References
-
- Azorlosa JL, Hartley NE, Deffner-Rappold C. Context-specific morphine tolerance and withdrawal: The effects of interdose interval. Psychobiology. 1994;22:304–311.
-
- Bao G, Kang L, Li H, Li Y, Pu L, Xia P, Ma L, Pei G. Morphine and Heroin Differentially Modulate In Vivo Hippocampal LTP in Opiate-Dependent Rat. Neuropsychopharmacology. 2007;32:1738–49. - PubMed
-
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 1988;244:126–32. - PubMed
-
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. - PubMed
-
- Bolger GT, Skolnick P, Rice KC, Weissman BA. Differential regulation of mu-opiate receptors in heroin- and morphine-dependent rats. FEBS Lett. 1988;234:22–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

