Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles
- PMID: 18472965
- PMCID: PMC2373913
- DOI: 10.1593/neo.08178
Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles
Abstract
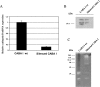
Vesicles shed by cancer cells are known to mediate several tumor-host interactions. Tumor microenvironment may, in turn, influence the release and the activity of tumor-shed microvesicles. In this study, we investigated the molecular mediators of the pH-dependent proinvasive activity of tumor-shed vesicles. Gelatinase zymography showed increased microvesicle activity of matrix metalloproteinases 9 and 2 as a result of acid exposure (pH 5.6) compared to pH 7.4. Thus, we reasoned that the cysteine protease cathepsin B might play a role in mediating the pH-dependent activation of gelatinases. Cathepsin B expression in tumor-shed microvesicles was confirmed by Western blot analysis and zymography. The activity of vesicle-associated cathepsin B measured using Z-Arg-Arg-pNA as substrate was significantly increased at acidic pH values. Inhibition of protease activity by the cysteine protease inhibitor, E-64, and treatment of ovarian cancer cells with small interfering RNA against cathepsin B suppressed the ability of tumor-shed microvesicles to stimulate both gelatinase activation and the invasiveness of endothelial cells observed at low pH values. We conclude that microvesicle shedding is a major secretory pathway for cathepsin B release from tumor cells. Hence, the acidic microenvironment found in most solid tumors may contribute to cathepsin B-mediated proinvasive capabilities of tumor-shed vesicles.
Figures


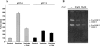

References
-
- Yan L, Shahied L, Tang Y, Kesavan P, Nakada MT. Tumor-host interactions. Preclinica. 2004;2:422–426.
-
- Zigrino P, Loffek S, Mauch C. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie. 2005;87:321–328. - PubMed
-
- Laconi E. The evolving concept of tumor microenvironments. Bioessays. 2007;29:738–744. - PubMed
-
- Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML. Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol. 1994;26:173–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
