Increased mtDNA levels without change in mitochondrial enzymes in peripheral blood mononuclear cells of infants born to HIV-infected mothers on antiretroviral therapy
- PMID: 18474497
- PMCID: PMC3895476
- DOI: 10.1310/hct0902-126
Increased mtDNA levels without change in mitochondrial enzymes in peripheral blood mononuclear cells of infants born to HIV-infected mothers on antiretroviral therapy
Abstract
Background: The effects of gestational nucleoside reverse transcriptase inhibitors (NRTIs) on mitochondrial DNA (mtDNA) are controversial. The effects of mtDNA depletion on mitochondrial function have not been assessed.
Method: In peripheral blood mononuclear cells (PBMCs) from infants born to HIV-infected women and infants born to HIV-1-uninfected women, mtDNA copy numbers were determined by quantitative PCR; nuclear (COXIV)- and mitochondrial (COXII)-encoded polypeptides of the oxidative phosphorylation enzyme cytochrome c-oxidase (COX or complex IV) were quantified by Western blot.
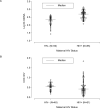
Results: Overall, 86 infants born to HIV-infected women and 50 controls were studied. HIV-infected mothers had a median CD4 count of 506 cells/microL; 59% had HIV RNA 50 copies/mL. No infant had clinical evidence of mitochondrial disease. The birth weight was lower (p = .016) and the body length higher (p = .002) in the HIV-exposed newborns. Eighty-one HIV-infected women had received gestational NRTIs (median duration 162 days). Median mtDNA copies/PBMC in the HIV-exposed infants were 505 (range, 120-1365) vs. 213 (27-426) in controls (p < .001). COX II/IV ratios were similar in both groups. Although mtDNA levels correlated inversely with maternal lactate, mitochondrial indices did not correlate with maternal CD4+ count, HIV RNA, smoking, or alcohol consumption.
Conclusion: We found elevated mtDNA copy numbers in PBMC of infants born to HIV-infected women, the majority of whom received NRTI-based therapy, when compared to those born to healthy HIV-negative controls, but there was no difference in mtDNA-encoded respiratory chain protein. The clinical consequence of these findings is unknown and requires further investigations.
Figures
References
-
- Venhoff N, Walker UA. Mitochondrial disease in the off-spring as a result of antiretroviral therapy. Expert Opin Drug Saf. 2006;5:373–381. - PubMed
-
- Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. - PubMed
-
- Gerschenson M, Erhart SW, Paik CY, St Claire MC, Nagashima K, Skopets B, et al. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3′-azido-3′-deoxythymidine. AIDS Res Hum Retroviruses. 2000;16:635–644. - PubMed
-
- Ewings EL, Gerschenson M, St Claire MC, Nagashima K, Skopets B, Harbaugh SW, et al. Genotoxic and functional consequences of transplacental zidovudine exposure in fetal monkey brain mitochondria. J Acquir Immune Defic Syndr. 2000;24:100–105. - PubMed
-
- Ekouevi DK, Toure R, Becquet R, Viho I, Sakarovitch C, Rouet F, et al. for the Agence Nationale de Recherches sur le SIDA 1201/1202 Ditrame Plus Study Goup Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV. Pediatrics. 2006;118:e1071–1077. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI36219/AI/NIAID NIH HHS/United States
- AI27664/AI/NIAID NIH HHS/United States
- AI27661/AI/NIAID NIH HHS/United States
- AI068636/AI/NIAID NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- AI25915/AI/NIAID NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- RR000080/RR/NCRR NIH HHS/United States
- AI25879/AI/NIAID NIH HHS/United States
- AI38558/AI/NIAID NIH HHS/United States
- RR00043/RR/NCRR NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- U01 AI027664/AI/NIAID NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- P30 AI042845/AI/NIAID NIH HHS/United States
- U01 AI025883/AI/NIAID NIH HHS/United States
- U01 AI025897/AI/NIAID NIH HHS/United States
- U01 AI025903/AI/NIAID NIH HHS/United States
- AI32907/AI/NIAID NIH HHS/United States
- AI42845/AI/NIAID NIH HHS/United States
- AI25897/AI/NIAID NIH HHS/United States
- RR00044/RR/NCRR NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- AI34853/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI27665/AI/NIAID NIH HHS/United States
- U01 AI025915/AI/NIAID NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- U01 AI032907/AI/NIAID NIH HHS/United States
- RR00069/RR/NCRR NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- RR00096/RR/NCRR NIH HHS/United States
- AI25883/AI/NIAID NIH HHS/United States
- AI25859/AI/NIAID NIH HHS/United States
- U01 AI025879/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U01 AI027665/AI/NIAID NIH HHS/United States
- AI25903/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials